POSITIVE
: Two (2) distinct colored lines appear. One line should be in the control region (C) and the other line should be in the test region (T).
NEGATIVE
: One (1) colored line appears in the control region(C). No apparent colored line appears in the test region (T).The negative result does not indicate the absence of analytes in the sample, it only indicates the level of tested analytes in the sample is less than cut-off level.
INVALID
: No colored lines appear or control line fails to appear, indicating that the operator error or reagent failure. Verify the test procedure and repeat the test with a new testing device.
- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
MR
-
Model Number:
-
HIV/HBSAG/HCV
-
Quality Certification:
-
ISO13485
-
Safety standard:
-
MSDS
-
Product name:
-
HIV/HBSAG/HCV
-
Format:
-
Strip/cassette
-
Dimension:
-
3.0mm/4.0mm
-
Specimen:
-
Whole blood/serum/plasma
-
Package:
-
25 pcs/box;50pcs/box
-
Storage:
-
4-30°C
-
Method:
-
Immunochromatography
-
MOQ:
-
1000pcs
-
Brand:
-
MR
Quick Details
-
Warranty:
-
2 years
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Shandong, China
-
Brand Name:
-
MR
-
Model Number:
-
HIV/HBSAG/HCV
-
Quality Certification:
-
ISO13485
-
Safety standard:
-
MSDS
-
Product name:
-
HIV/HBSAG/HCV
-
Format:
-
Strip/cassette
-
Dimension:
-
3.0mm/4.0mm
-
Specimen:
-
Whole blood/serum/plasma
-
Package:
-
25 pcs/box;50pcs/box
-
Storage:
-
4-30°C
-
Method:
-
Immunochromatography
-
MOQ:
-
1000pcs
-
Brand:
-
MR
Product Description

Specification
|
item
|
value
|
|
Place of Origin
|
China
|
|
|
Shandong
|
|
Brand Name
|
MR
|
|
Model Number
|
HIV
|
|
Power Source
|
Manual
|
|
Warranty
|
2 years
|
|
After-sale Service
|
Online technical support
|
|
Material
|
plastic, paper
|
|
Shelf Life
|
2 years
|
|
Quality Certification
|
ISO13485
|
|
Instrument classification
|
Class II
|
|
Safety standard
|
MSDS
|
|
Product name
|
HIV/HBSAG/HCV rapid test
|
|
Format
|
Strip/Triple line cassette
|
|
Dimension
|
3.0mm/4.0mm
|
|
Specimen
|
Whole blood/serum/plasma
|
|
Package
|
25 pcs/box;50pcs/box
|
|
Storage
|
4-30°C
|
|
Shelf Life
|
24months
|
|
Method
|
Immunochromatography
|
|
MOQ
|
1000pcs
|
|
Brand
|
MR
|
Intended Use
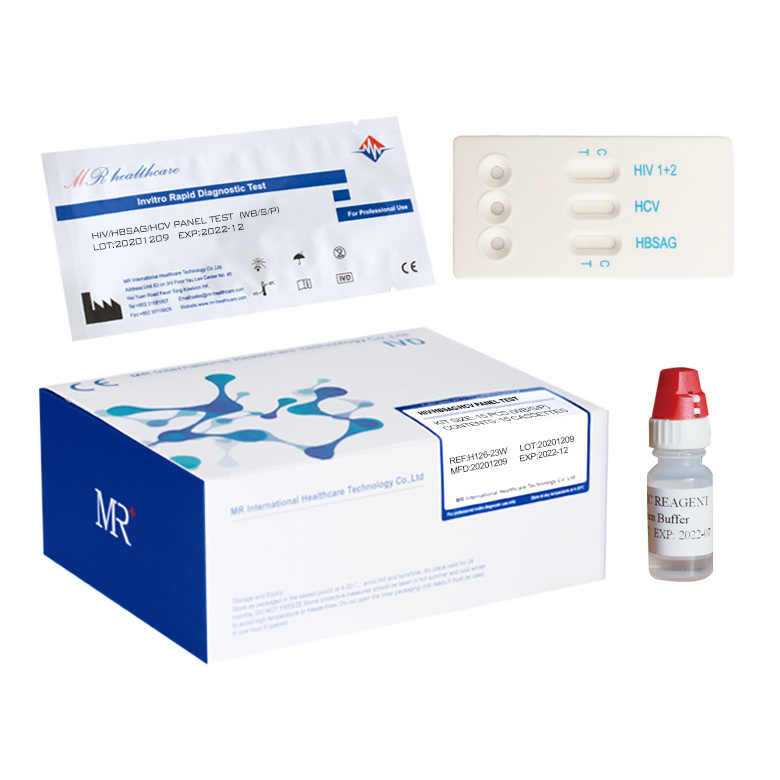
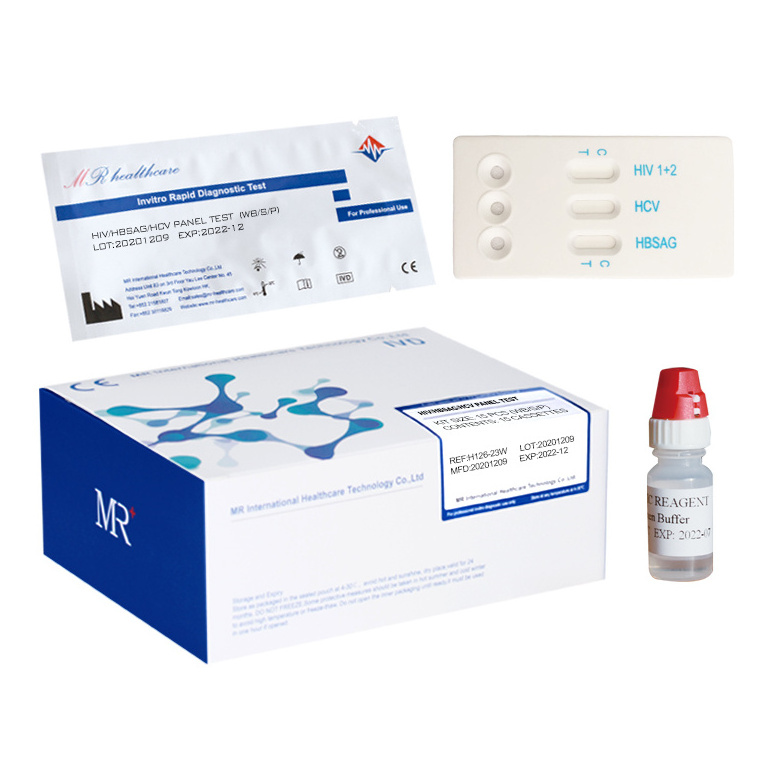
The reagent is used to detect the Hepatitis C Virus Antibody in serum/plasma/whole blood qualitatively.
Test procedure
1. Instruction for Use must be read entirely before taking the test. Allow the test device controls to equilibrate to room
temperature for 30 minutes (20°C-30°C) prior to testing. Do not open the inner packaging until ready, it must be used in one hour if opened.
2. 2. Test Procedure
Strip:
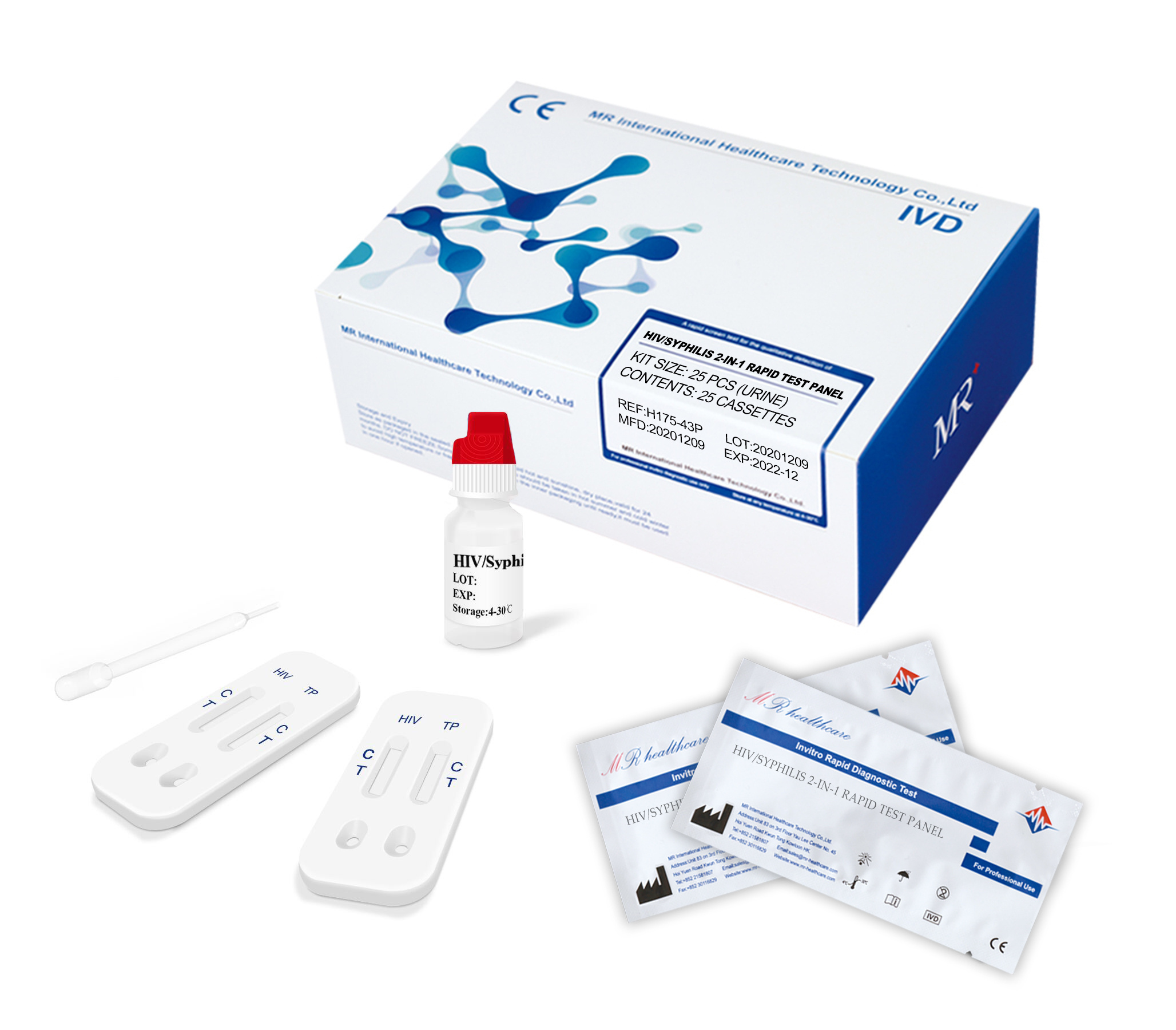
(1) Remove the test strip from the sealed pouch, place it on a clean and level surface with the sample adding area up.
(2) Using the dropper, vertically transfer one(1) drop (25µl) of serum/plasma/whole blood sample onto the sample adding area of the strip, avoiding the formation of bubbles. Add two(2) drops (80-100ul) of sample buffer onto the sample adding area of the strip.
(3) Observe the test results immediately within 10-20 minutes, the result is invalid over 20 minutes.
Cassette:
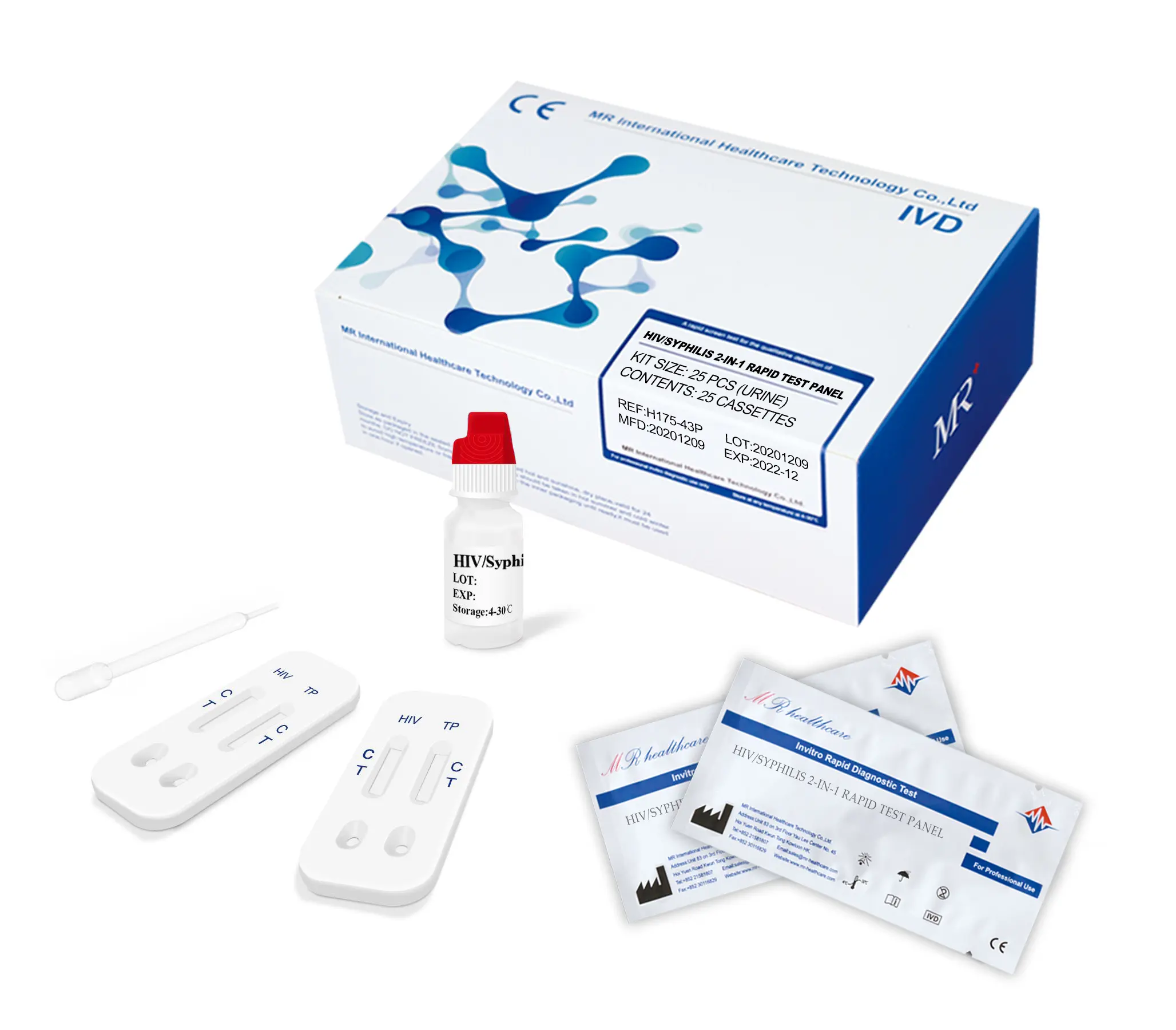
* (1) Remove the test cassette from the sealed pouch, place it on a clean and level surface with the sample well up.
* (2) Using the dropper, vertically transfer one(1) drop (25µl) of serum/plasma/whole blood sample into the sample well (S) of the cassette, avoiding the formation of bubbles. Add two(2) drops (80-100ul) of sample buffer into the sample well of the cassette.
(3) Observe the test results immediately within 10-20 minutes, the result is invalid over 20 minutes.
* Don't read result after 20 minutes. To avoid confusion, discard the test device after interpreting the result.
temperature for 30 minutes (20°C-30°C) prior to testing. Do not open the inner packaging until ready, it must be used in one hour if opened.
2. 2. Test Procedure
Strip:
(1) Remove the test strip from the sealed pouch, place it on a clean and level surface with the sample adding area up.
(2) Using the dropper, vertically transfer one(1) drop (25µl) of serum/plasma/whole blood sample onto the sample adding area of the strip, avoiding the formation of bubbles. Add two(2) drops (80-100ul) of sample buffer onto the sample adding area of the strip.
(3) Observe the test results immediately within 10-20 minutes, the result is invalid over 20 minutes.
Cassette:
* (1) Remove the test cassette from the sealed pouch, place it on a clean and level surface with the sample well up.
* (2) Using the dropper, vertically transfer one(1) drop (25µl) of serum/plasma/whole blood sample into the sample well (S) of the cassette, avoiding the formation of bubbles. Add two(2) drops (80-100ul) of sample buffer into the sample well of the cassette.
(3) Observe the test results immediately within 10-20 minutes, the result is invalid over 20 minutes.
* Don't read result after 20 minutes. To avoid confusion, discard the test device after interpreting the result.
Result Judgment
Manufacturing procedure
Packing & Delivery
Hot Searches