- Product Details
- {{item.text}}
Quick Details
-
Size:
-
maximum 190mL
-
Blood compartment volume (Type A):
-
60≤V≤100ml
-
Blood compartment volume (Type B):
-
110≤V≤150ml
-
Blood compartment volume (Type C):
-
150≤V≤190ml
-
Adsorbent volume (Type A):
-
150±15
-
Adsorbent volume (Type B):
-
250±15
-
Adsorbent volume (Type C):
-
350±15
-
Blood compartment volume:
-
See 1.4
-
UV absorbance:
-
Less than 0.1
-
Sterile:
-
The column is sterile
-
Bacterial endotoxin:
-
Less than 0.5EU/mL
Quick Details
-
Place of Origin:
-
Guangdong, China
-
Brand Name:
-
MSL
-
Model Number:
-
MSLGL08
-
Size:
-
maximum 190mL
-
Blood compartment volume (Type A):
-
60≤V≤100ml
-
Blood compartment volume (Type B):
-
110≤V≤150ml
-
Blood compartment volume (Type C):
-
150≤V≤190ml
-
Adsorbent volume (Type A):
-
150±15
-
Adsorbent volume (Type B):
-
250±15
-
Adsorbent volume (Type C):
-
350±15
-
Blood compartment volume:
-
See 1.4
-
UV absorbance:
-
Less than 0.1
-
Sterile:
-
The column is sterile
-
Bacterial endotoxin:
-
Less than 0.5EU/mL


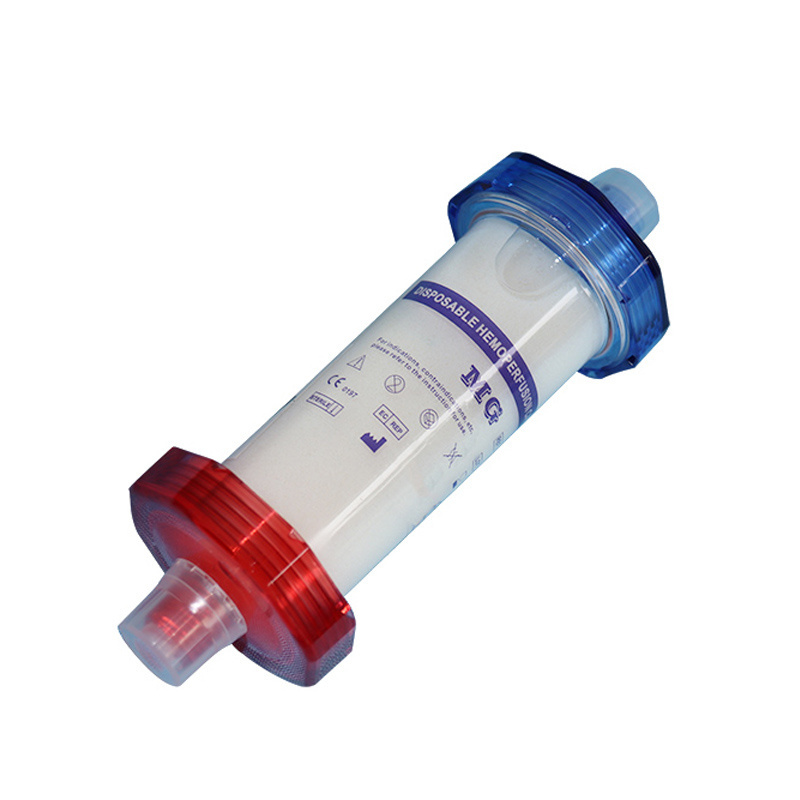
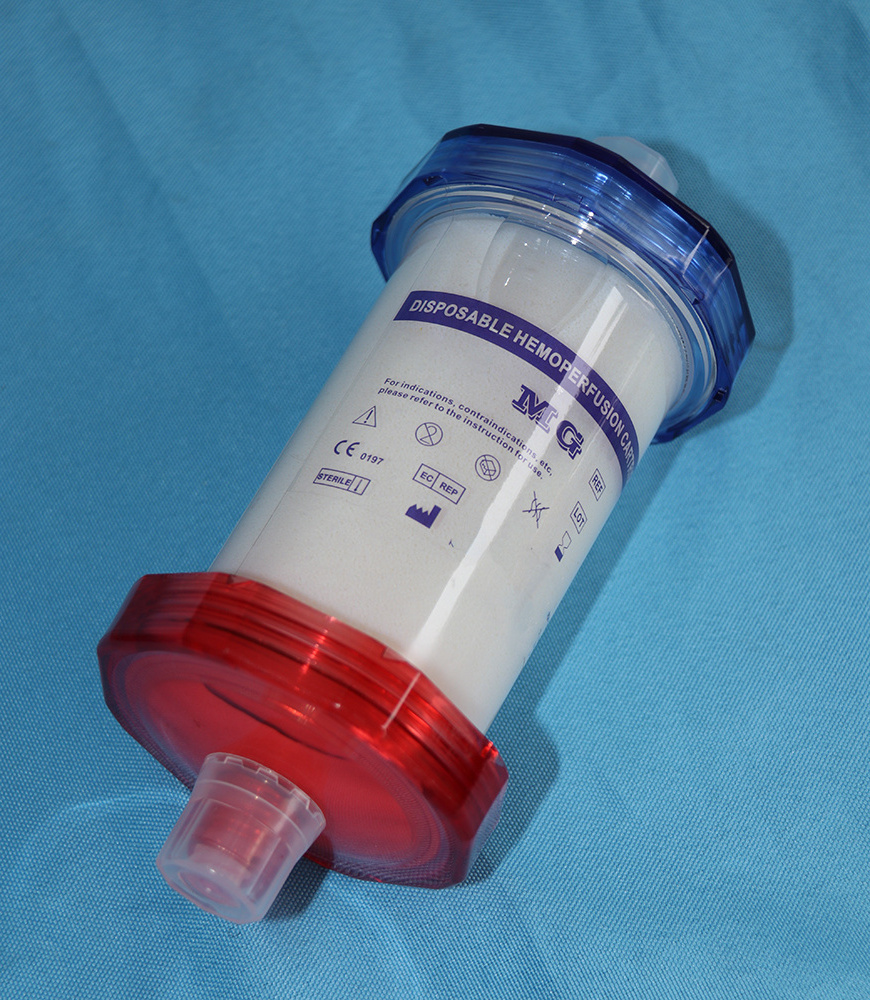
Introduction of MG Therapy
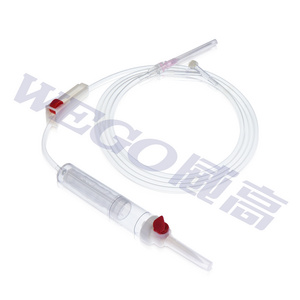
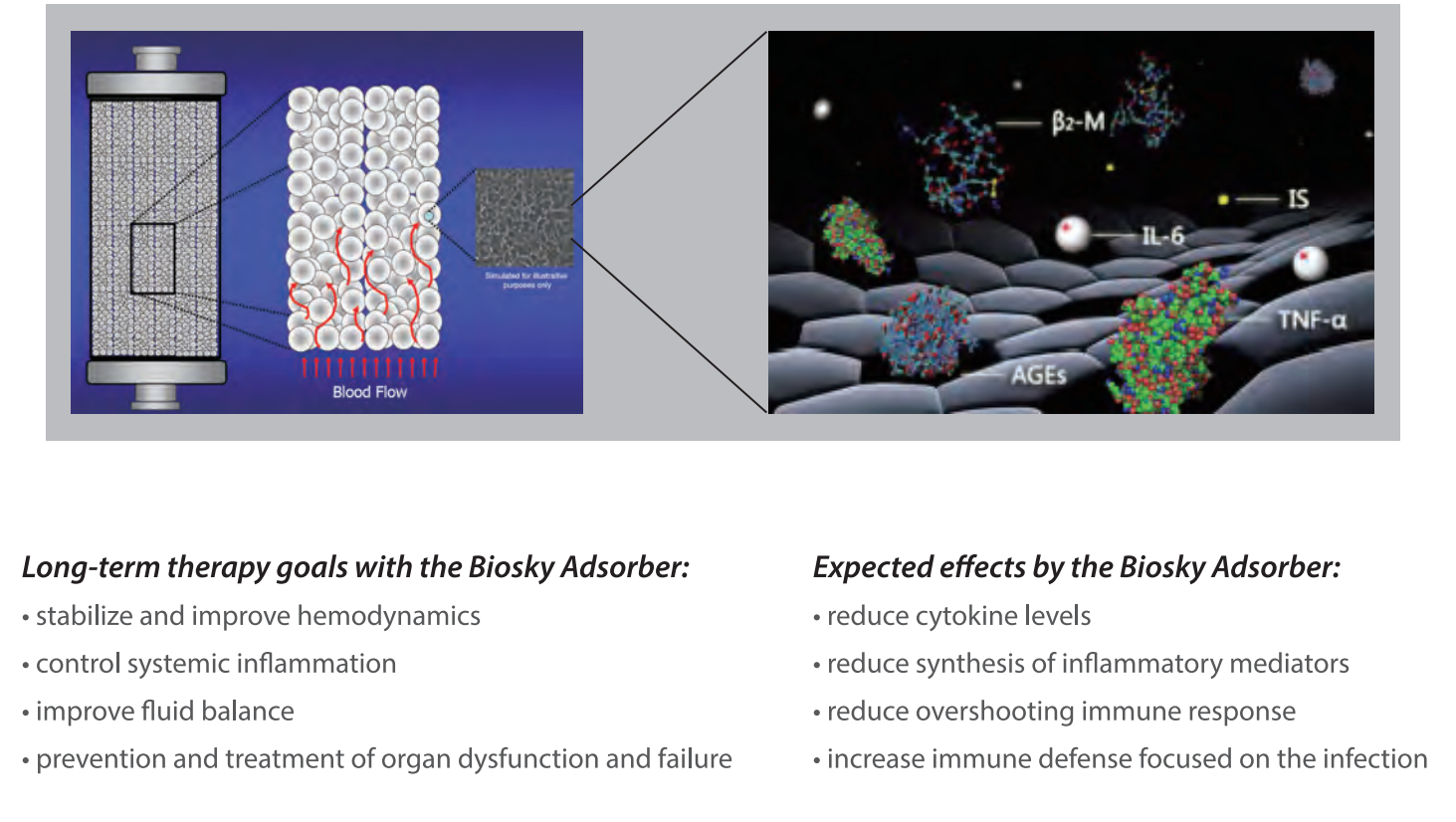
The main focus of MG therapy is the removal of inflammatory mediators, particulary cytokines and chemokines, from the patient's blood. The MG cartridge removes a vide range of inflammatory mediators between a 10-63 kDa molecular weight range, where most cytokines can be found. Standard high-flux dialysis or CRRT is only capable of removing substances generally below 15kDa in size.
As a result of the MG therapy, life-threatening complications associated with the cytoline storm can be avoided, and stabilization after a hyperinflammatory phase improved.
Mechanism
The mechanism of hemoperfusion is based on biocompatible and highly porous polymer beas which allow to remove a wide range of toxic substances such from the blood, such as drugs, cytokines and toxins, by combining functions of the pore structure, Van der Walls Force and hydrogen bond.
- blood flow 180-400ml/min
- pre-filled with water for injection
- steam sterilized, 3 years shelf life
- size selectivity <63 kDa
- low flow resistance
- blood volume 150-190ml
1.Product Specification
Product specifications are named after adsorbent volume (mL).
|
Specification |
Blood compartment volume ( mL ) |
Adsorbent volume ( mL ) |
|
MSLGL08A |
60≤V≤100 |
150± 15 |
|
MSLGL08B |
110≤V≤150 |
250± 15 |
|
MSLGL08C |
150≤V≤190 |
350± 15 |
2. Functional description
2.1 Appearance: The shell of the adsorption column should be transparent, the inner and outer
shell surface is smooth.
2.2 Blood compartment volume: See 1 .4
2.3 Particle shedding: In the 100mL test solution of the adsorption column, and the number of the
15 um ~ 25 um particles is less than 200, and the number of particles greater than 25um in the
100mL test solution is less than 100.
2.4 Reducing material: 20mL test solution and [c (KMnO 4 ) =0.002mol/L] of the same batch of blank
solution should be no more than 2.0mL
2.5 Metal ion: When using a colorimetric assay, showing the test liquid color should not exceed the
concentration of c(Pb 2+ ) =1 ug/mL standard solution
2.6 pH value: The difference between the pH value of the test solution and the blank control
solution should be no more than 1.5
2.7 Evaporation residue: The total amount of evaporation residue in 50mL test liquid should be no
more than 2mg
2.8 UV absorbance: Less than 0.1
2.9 Sterile: The column is sterile
2.10 Bacterial endotoxin: Less than 0.5EU/mL
2.11 Sealing performance: The positive pressure in the blood compartment of the adsorption
column is 100kPa.
2.12 Adsorption property:
Adsorption properties of pentobarbital sodium: Drop rate of concentration is no less than 80%
Adsorption properties of creatinine: Drop rate of concentration is no less than 15%
Adsorption properties of Vitamin B12: Drop rate of concentration is no less than 80%
Adsorption properties of β 2-microglobulin: The adsorption capacity is more than 0.3mg/mL
Adsorption properties of parathyroid hormone (PTH): The adsorption capacity is more than
0.4pmol/mL
2.13 Temperature resistance: The adsorption column should not be deformed and cracked in the
range of 0 ~50 º C.
3. Classification
Class IIb, according to MDD Annex IX , rule 3, “ All non-invasive devices intended for
modifying the biological or chemical composition of blood, other body liquids or other liquids
intended for infusion into the body are in Class IIb ”