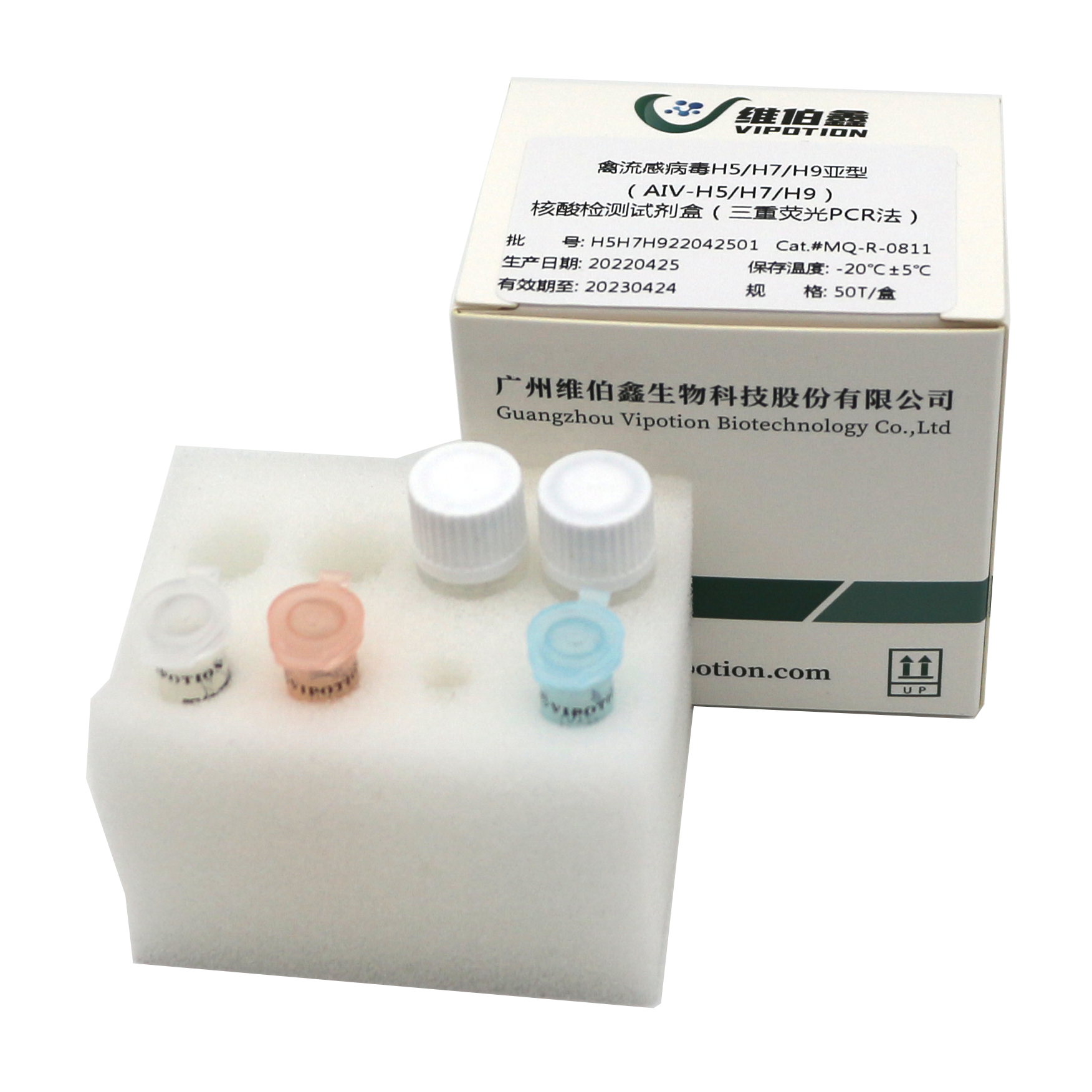
According to the HA gene of H5/H7/H9 subtype of avian influenza virus, this kit designs specific primers and probes respectively, and uses one-step fluorescent RT-PCR technology to amplify and detect the RNA of H5/H7/H9 subtype of avian influenza virus in vitro, which is used for clinical etiological diagnosis of suspected infected materials.
- Product Details
- {{item.text}}
Quick Details
-
Size:
-
6cm*4.5cm*6cm
Quick Details
-
Place of Origin:
-
Guangdong, China
-
Brand Name:
-
VIUICK
-
Model Number:
-
MQ-R-0811
-
Size:
-
6cm*4.5cm*6cm
Product Description
Avian influenza is a poultry infectious disease caused by avian influenza virus (AIV). Chickens, turkeys, ducks, quails and other poultry can be infected. The incidence varies greatly, with some causing acute death and some being asymptomatic.
AIV has strong antigenic variability, a wide host range, and no cross-protection between subtypes, resulting in frequent avian influenza outbreaks. Highly pathogenic avian influenza is mainly caused by H5 or H7 subtype AIV. It has a very short incubation period, sudden outbreak, and morbidity and mortality rates as high as 100%. Currently, the prevalence of low-to-medium pathogenic avian influenza, mainly H9 subtype, in some areas of China has caused great economic losses to the poultry industry.
Early and rapid detection is undoubtedly the key to preventing and controlling avian influenza.
Product
Principle
|
Contents
|
48 Tests/Kit
|
|
AIV-H5/H7/H9 PCR Master Mix
|
Lyophilized powder ×1 bottle
|
|
Redissolved Diluent
|
1.0mL×2 Tube
|
|
AIV-H5/H7/H9 Positive Control
|
Lyophilized powder ×1 Tube
|
|
AIV-H5/H7/H9 Negative Control
|
1.0mL×1 Tube
|
Product Features:
1.
Accurate and rapid virus detection with high sensitivity.
2.
Lyophilized bottles are packed to avoid errors in experimental results caused by repeated freezing and thawing, and to avoid the loss of highly viscous components such as enzymes.
3.
Transported and stored at room temperature, it has extremely strong stability withstanding high temperatures of 55°C for 30 days. It does not require cold chain and reduces transportation costs.
4.
One-step use, no need to wait for room temperature to melt, no system configuration required, just add the appropriate amount of nucleic acid.
|
Specification
|
48 Tests/Kit
|
|
Storage Conditions and Term of Validity
|
Store below 30°C. It is valid for 12 months.
|
|
Sample Requirements
|
Oropharyngeal swab or cloacal swab
|
|
Amplification Instrument
|
Real-time fluorescence PCR instrument with FAM channel.
|

OEM Bulked Package Supported
Packing & Delivery


Hot Searches