- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
test right
-
Model Number:
-
02
-
Power Source:
-
test kit rapid
-
Safety standard:
-
test kit rapid
-
Product name:
-
Antigen Rapid Test
-
Specimen:
-
Whole Blood
-
Format:
-
Strip Cassette
-
Certificate:
-
CE ISO
-
MOQ:
-
1000pcs
-
Packing:
-
25 Kit/box
-
Storage:
-
Room Tempreture
-
Sensitivity:
-
High Accuracy 99.99%
-
Method:
-
Colloidal Gold Rapid Test
Quick Details
-
Warranty:
-
2 years
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Henan, China
-
Brand Name:
-
test right
-
Model Number:
-
02
-
Power Source:
-
test kit rapid
-
Safety standard:
-
test kit rapid
-
Product name:
-
Antigen Rapid Test
-
Specimen:
-
Whole Blood
-
Format:
-
Strip Cassette
-
Certificate:
-
CE ISO
-
MOQ:
-
1000pcs
-
Packing:
-
25 Kit/box
-
Storage:
-
Room Tempreture
-
Sensitivity:
-
High Accuracy 99.99%
-
Method:
-
Colloidal Gold Rapid Test
One Step HAV IgM Rapid Screen Test
The quick one-step test utilizes a sandwich immunoassay system and the immunochromatographic detection assay, to be performed in one assay. If HAV antibody is present in the sample in concentration above the detection, a labeled antigen-dye complex will be formed. This complex is then captured by antibody immobilized in the Test Zone of the membrane, producing a visible pink-rose color band on the membrane. The color intensity will depend on the concentration of HAV antibody in the sample.
HBV Combo Rapid test
Qualitative detection of the five Hepatitis B markers HBsAg, HBsAb,HBeAg,HBeAb,HbcAb in human serum (or plasma),for clinical diagnosis.
HCV Ab Rapid Test (Serum/Plasma/Whole Blood)
The HCV Ab Rapid Test is an indirect lateral flow chromatographic immunoassay for the qualitative detection of IgG and IgM anti-Hepatitis C virus (HCV) in human serum,plasma or whole blood. It is intended to be used as a screening test and as an aid in the diagnosis of infection with HCV.Any reactive speciment with the HCV ab rapid test must be confirmed with alternative testing method(s) and clinical findings.
Hepatitis E virus IgM test Cassette
The Hepatitis E virus IgM Test is a lateral flow immunoassay for the simultaneous detection and differentiation of HEV IgM in human serum, plasma, or whole blood. It is intended to be used as a screening test and as an aid in the diagnosis of infection with HEV. Any reactive specimen with the Hepatitis E virus IgM Rapid Test must be confirmed with alternative testing method(s) such as ELISA or PCR.
| Format | Strip/Cassette |
| Specimen | WB/Serum/Plasma |
| Packaging | 40 pcs in one box, 2000 pcs in one carton |
| MOQ | 500 pcs |
| Certificate | CE/ISO |
HAV Test Method And Result
1. Dilute the serum (or plasma) specimen 1000times with dilute.
2. Open a pouch containing a cassette, remove the test kit from the pouch and place it horizontally on the desk.
3. Pipette 2 or 3 drops of diluted specimen into the sample well of the cassette.
4. Read results within 10-15 minutes. Do not read results after 20 minutes.

Negative:
Only one pink band appears on test region of the Cassette. This indicates that there is no detectable anti-HAV IgM in the serum.
Positive:
Two pink bands appear on test region of the Cassette. This indicates that the
specimen contains detectable amount of anti-HAV IgM.
Invalid:
If without colored band appears on test region, this is an indication of apossible error in performing the test. The test should be repeated using a new device.
HBV Test Method And Result
1. Revert the test board and the testing samples to romm temperature(20-30℃)
2.The right side of the test board should be kept horizontal from the original package, from left to right, respectively corresponding to HBsAg,HBsAb,HBeAg,HBeAb,HBcAb.With a samll straw to take subjects' serum,and add into 5 sample wells of the test board by drops(70Ul per well or 2 drops)
3.Observe and record the experimental result within 15 mins.Weekly positive samples appear test line in 15-20 mins.Determination after 30 mins is invalid.
Note:Take out test paper from the original packaging, and it should be used within 1 hour as soon as possible,expecially at room temperature above 30℃ or in a highly humid environment.
1. HBsAg HBsAb, HBeAg (Sandwich method)

Negative:Only one purple bar (control line) in the control C zone.
Positive:Detecting T zone there two purple bars in the control C zone.
Invalid:Detecting T zone there is no purple bar in the control C zone.
2.HBeAb,HBcAb(Competition method)
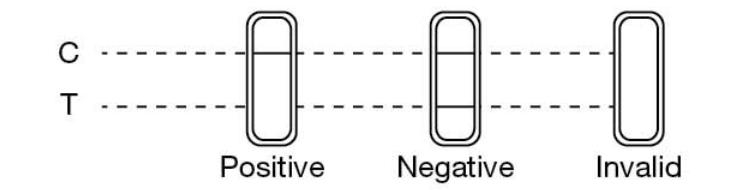
Negative:Detecting T zone there are two purple bars in the control C zone.
Positive:Only one purple bat (control line) in the control C zone.
Invalid:Detecting T zone there is no purple bar in the control C zone.