- Product Details
- {{item.text}}
Quick Details
-
Variety:
-
Horse Chestnut Extract
-
Place of Origin:
-
Hunan, China
-
Brand Name:
-
MISAL
-
Model Number:
-
MS-NP-0389
-
Appearance:
-
Brown yellow fine powder
-
Test Method:
-
HPLC UV
-
Active Ingredient:
-
Aescin
-
CAS:
-
6805-41-0
-
Molecular Formula:
-
C55H86O24
-
Molecular Weight:
-
1131.26
-
Shelf Life:
-
24 Months
Quick Details
-
Form:
-
Powder
-
Extraction Type:
-
Solvent Extraction
-
Grade:
-
Top Grade
-
Variety:
-
Horse Chestnut Extract
-
Place of Origin:
-
Hunan, China
-
Brand Name:
-
MISAL
-
Model Number:
-
MS-NP-0389
-
Appearance:
-
Brown yellow fine powder
-
Test Method:
-
HPLC UV
-
Active Ingredient:
-
Aescin
-
CAS:
-
6805-41-0
-
Molecular Formula:
-
C55H86O24
-
Molecular Weight:
-
1131.26
-
Shelf Life:
-
24 Months
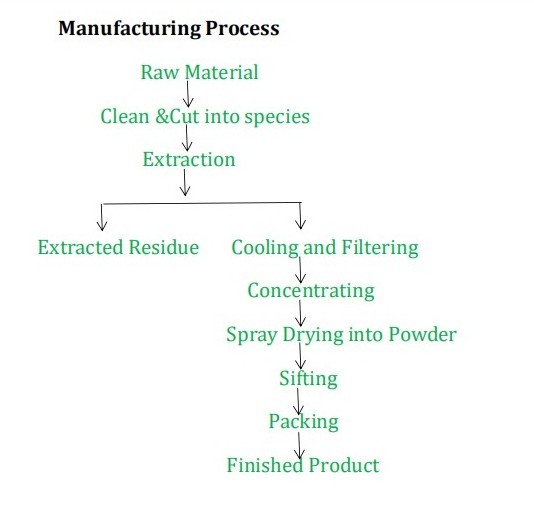
Natural supplement Aescin 20% Horse Chestnut Extract

|
1. Product name: Horse chestnut Extract |
|
2. Latin name: Aesculus Hippocastanum L. |
|
3. Specification: 20%-98% Aescin by UV , 98% Esculin HPLC |
|
4 . Appearance: Brown yellow fine powder |
|
5 . MOQ: 10G |
|
6 . Normal Package: 1KG/Aluminum foil bag, 25kg/Fiber drum |
|
7 . Normal Payment: T/T, L/C, Paypal, Western Union, Trade Assurance |
|
8 . Lead Time:Based on your required quantity |
What is Horse Chestnut extract ?
Such years of pharmacological research and clinical application, which proved that Aescinate applied to play an anti-inflammatory, anti-oxidation role and keep moisturizing. In terms of relative sodium aescinate, aescin has a great significant treatment effect on the human body and few side effects. It can replace sodium aescinate in cosmetics and medicines.
Function:
2.Decreases edema -- swelling caused by accumulation of fluid in the veins.
3.Reduces venous inflammation.
4.Stimulates circulation.
5.Treats hemorrhoids (traditional).
|
Certificate of Analysis |
||
|
Analysis |
Specification |
Result |
|
Physical Description |
||
|
Appearance |
Brown Yellow powder |
Complies |
|
Odor |
Characteristic |
Characteristic |
|
Particle size |
100% pass 80 mesh |
Complies |
|
Chemical Tests |
||
|
Identification |
Must Positive |
Complies |
|
A ssay |
>20% |
22.45% |
|
Loss on drying |
5.0% Max |
1.92% |
|
Heavy metals |
10ppm Max |
Complies |
|
As |
2ppm Max |
Complies |
|
Pb |
2ppm Max |
Complies |
|
Microbiology Control |
||
|
Total plate count |
1000cfu/g Max |
Complies |
|
Yeast & Mold |
100cfu/g Max |
Complies |
|
E. Coli |
Negative |
Negative |
|
Salmonella |
Negative |
Negative |
|
Conclusion |
Complies with the standards. |
|
Application:
1
. Functional food as capsules or pills;
2. Water-soluble beverages;
3. Health products as capsules or pills