- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
OEM/SoyMed
-
Model Number:
-
HCV
Quick Details
-
Warranty:
-
1 Year
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Zhejiang, China
-
Brand Name:
-
OEM/SoyMed
-
Model Number:
-
HCV
Product Description
Intended Use:
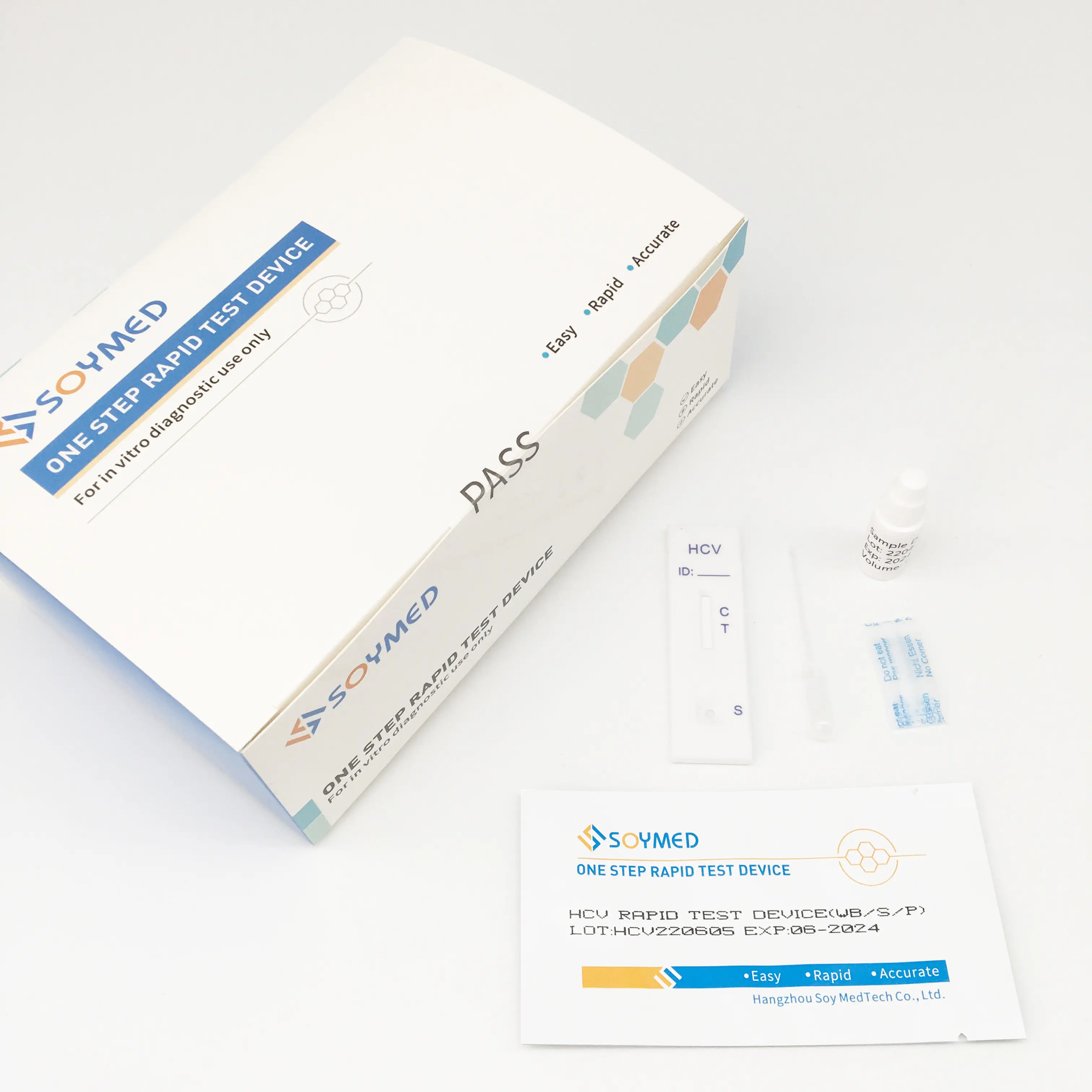
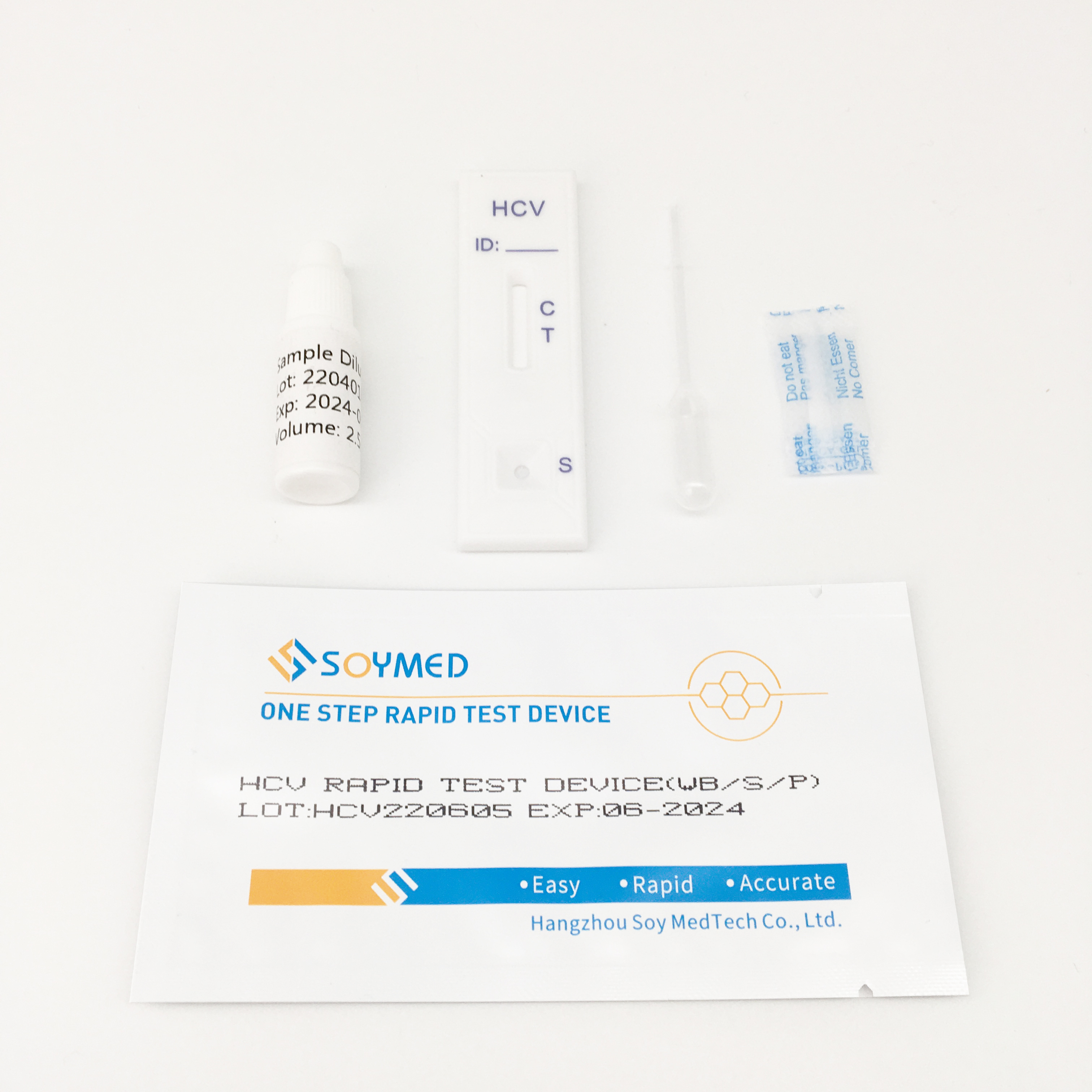
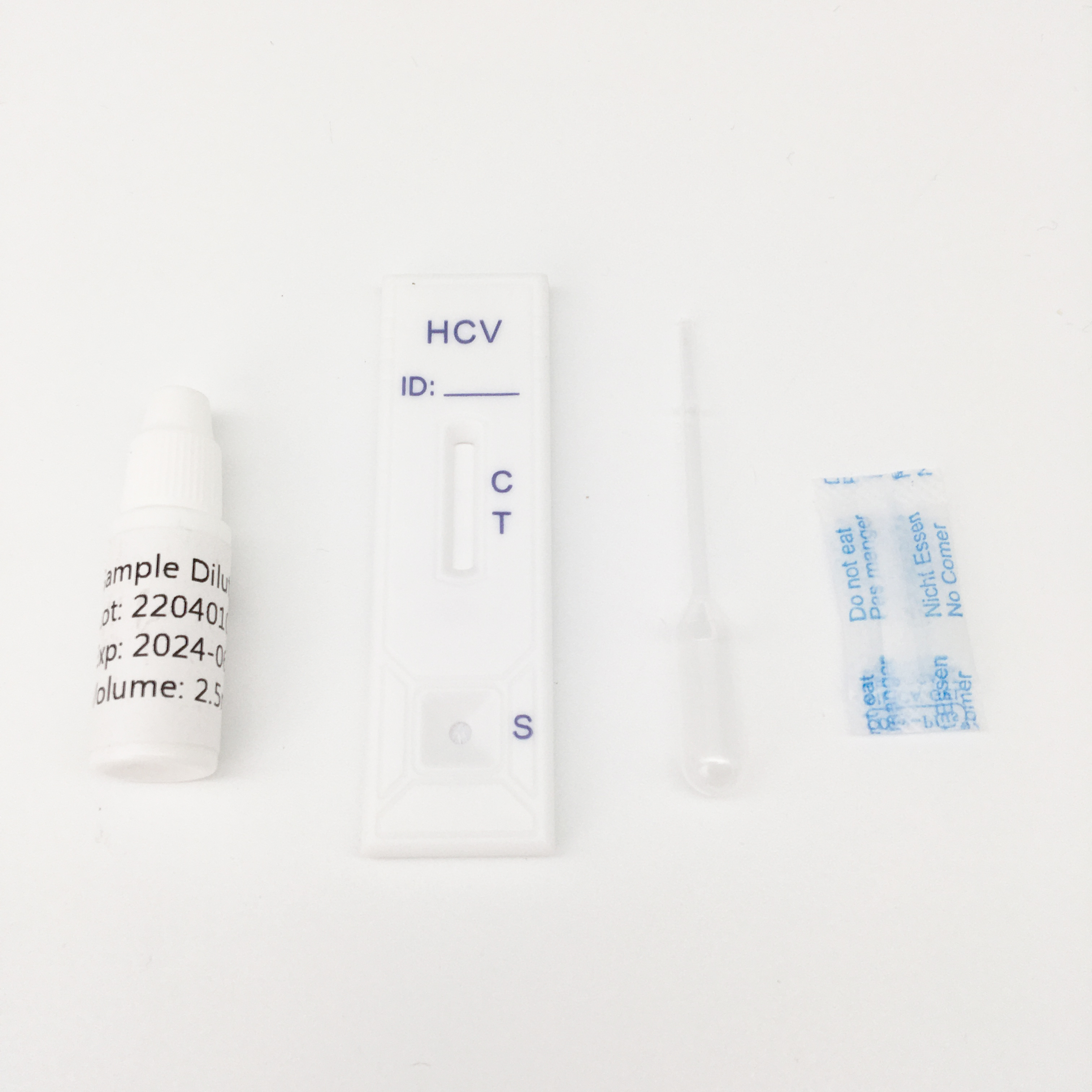
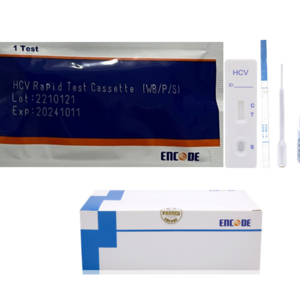
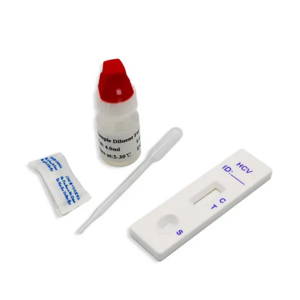
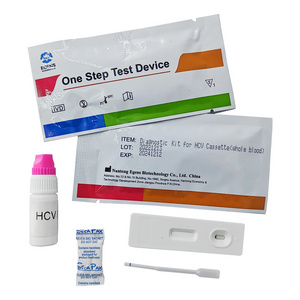
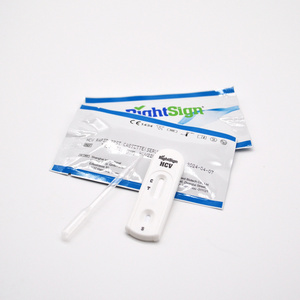
HCV Rapid Test Kit/Medical Diagnostic Rapid Test Device HCV with CE Approved is a rapid visual immunoassay for the qualitative presumptive detection of antibodies to HCV in human serum or plasma specimens. This kit is intended to be used as an aid in the diagnosis of HCV infection.
1. Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA Virus. Antibody to HCV is found in over 80% of patients with well-documented non-A, non-B hepatitis. Conventional methods fail to isolate the virus in cell culture or visualize it by electron microscope. Cloning the viral genome has made it possible to develop serologic assays that use recombinant antigens
2. Compared to the first generation HCV EIAs using single recombinant antigen, multiple antigens using recombinant protein and/or synthetic peptides have been added in new serologic tests to avoid nonspecific cross-reactivity and to increase the sensitivity of the HCV antibody tests
3. The Hepatitis C Virus Antibody Rapid Testing kit is a rapid test to qualitatively detect the presence of antibody to HCV in a serum or plasma specimen. The test utilises a combination of protein A coated particles and recombinant HCV proteins to selectively detect antibody to HCV in serum or plasma.
4. The recombinant HCV proteins used in the test are encoded by the genes for both structural (nucleocapsid) and non-structural proteins.
1. Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA Virus. Antibody to HCV is found in over 80% of patients with well-documented non-A, non-B hepatitis. Conventional methods fail to isolate the virus in cell culture or visualize it by electron microscope. Cloning the viral genome has made it possible to develop serologic assays that use recombinant antigens
2. Compared to the first generation HCV EIAs using single recombinant antigen, multiple antigens using recombinant protein and/or synthetic peptides have been added in new serologic tests to avoid nonspecific cross-reactivity and to increase the sensitivity of the HCV antibody tests
3. The Hepatitis C Virus Antibody Rapid Testing kit is a rapid test to qualitatively detect the presence of antibody to HCV in a serum or plasma specimen. The test utilises a combination of protein A coated particles and recombinant HCV proteins to selectively detect antibody to HCV in serum or plasma.
4. The recombinant HCV proteins used in the test are encoded by the genes for both structural (nucleocapsid) and non-structural proteins.

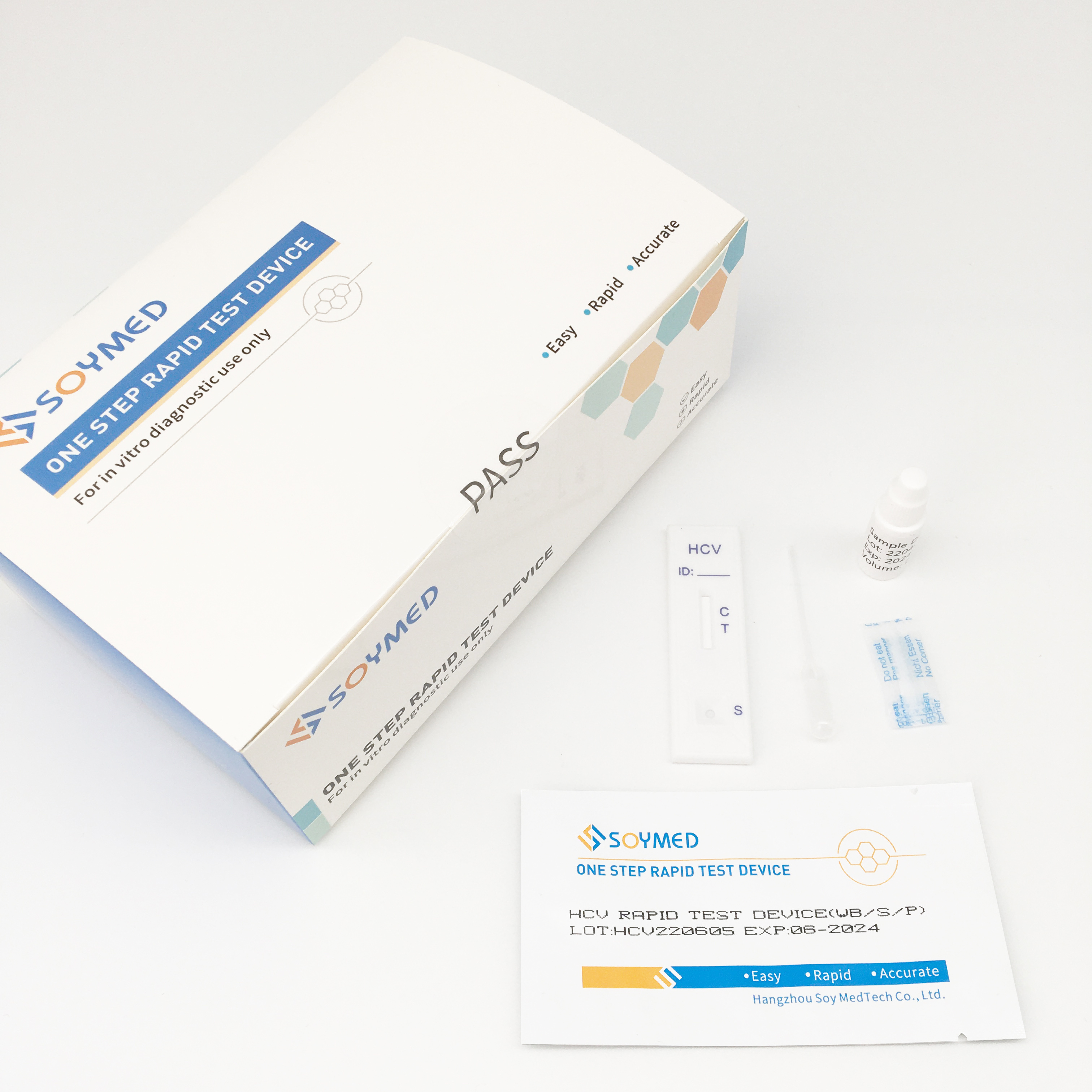
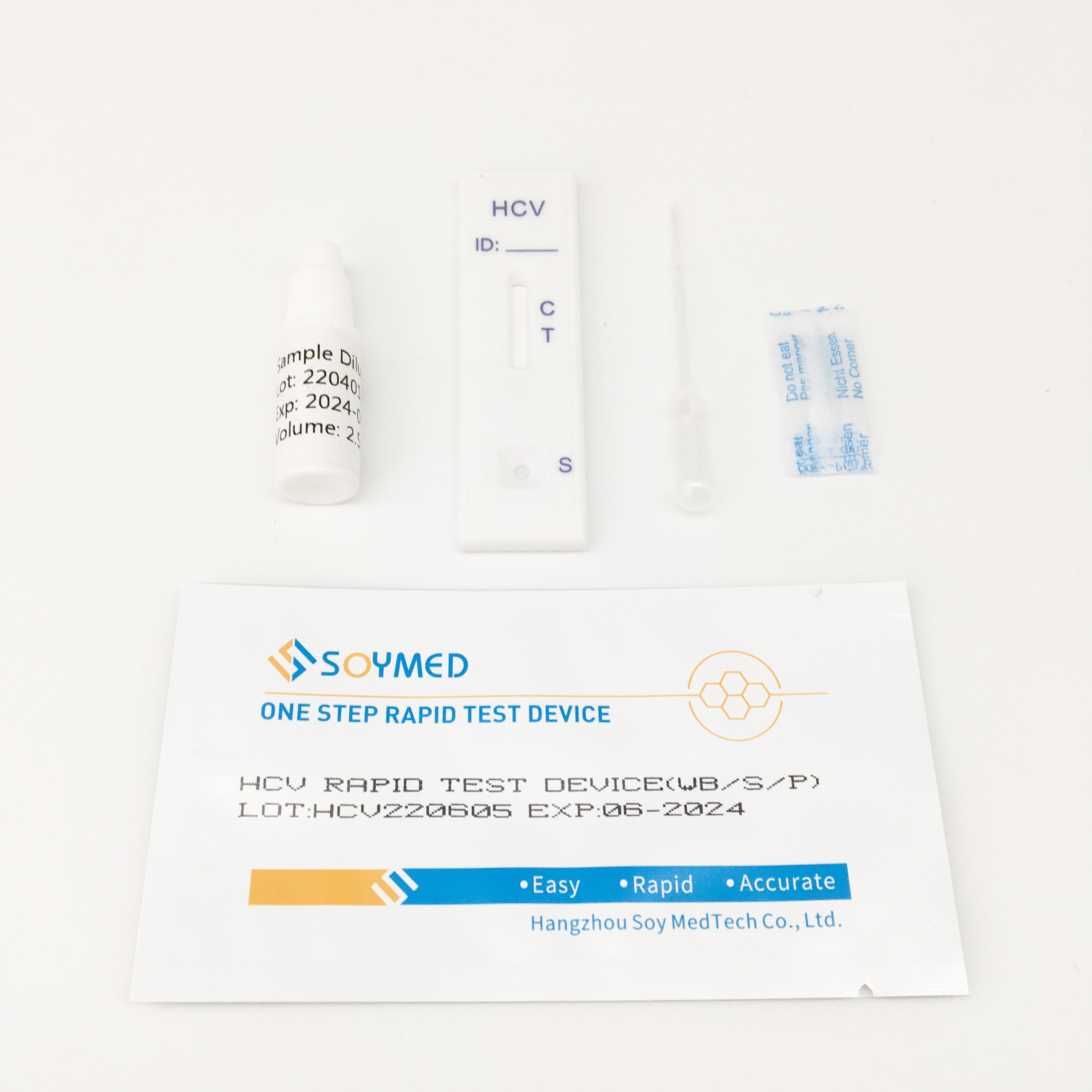
Specification
|
Test
|
96
|
|
Method
|
ELISA method: Enzyme Linked Immunosorbent Assay
|
|
Principle
|
Indirect ELISA: Antigen Coated Plate
|
|
Detection Range
|
Qualitative: Positive & Negative Control
|
|
Sample
|
10ul
|
|
Specificity
|
99.55%
|
|
Sensitivity
|
100%
|
|
Total Time
|
15 min
|
|
Shelf Life
|
12 Months from the manufacturing date
|
Hot Searches