- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
MR
-
Model Number:
-
combo-WSP-S
-
Quality Certification:
-
CE
-
Safety standard:
-
ISO14385
-
Product name:
-
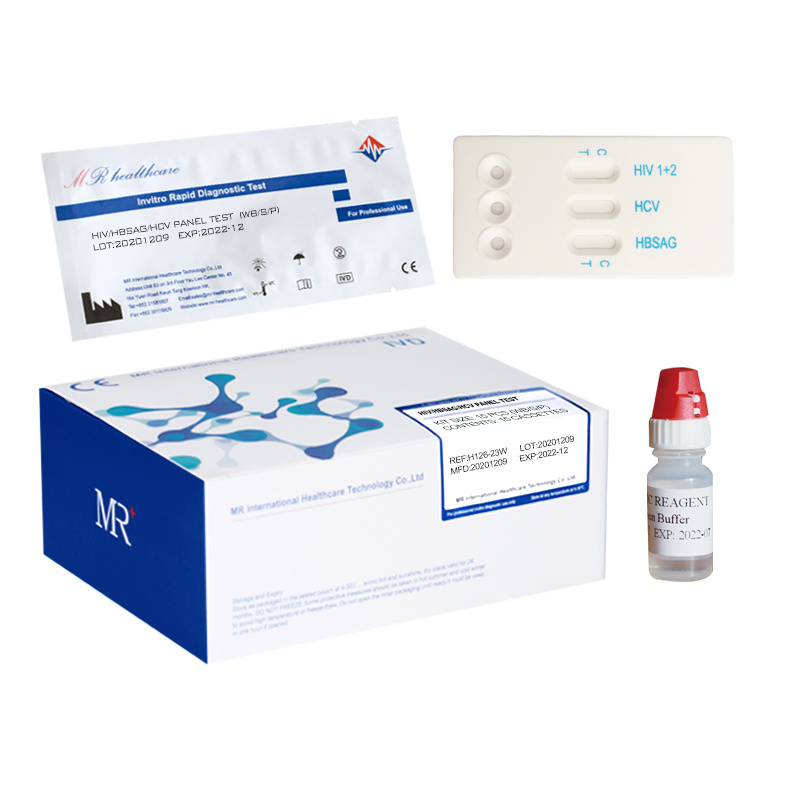
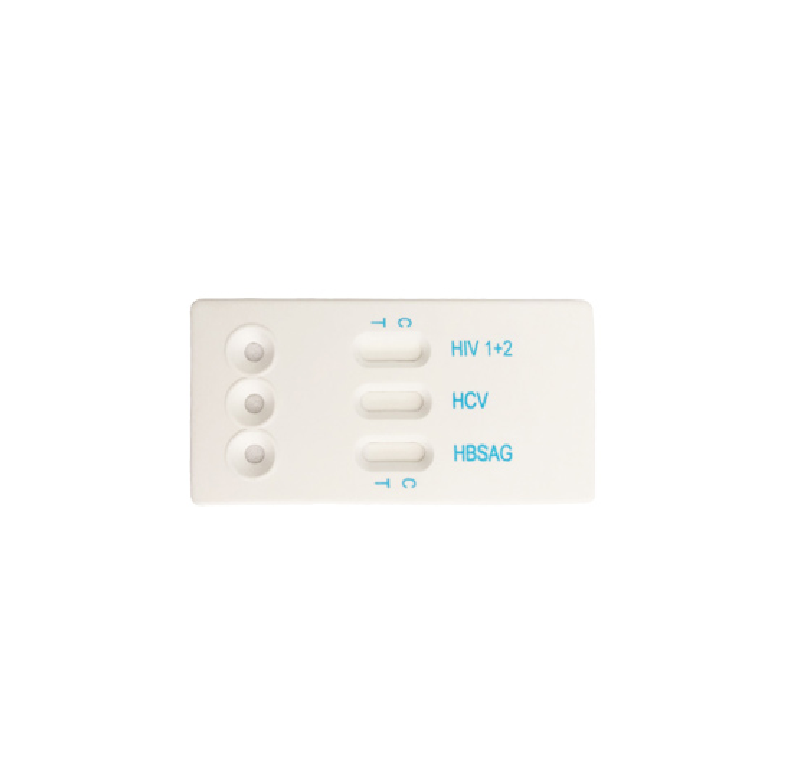
HIV HCV HBsAg TP combo cassette
-
Format:
-
cassette
-
Dimension:
-
3.0mm
-
Specimen:
-
whole blood/serum/plasma
-
Package:
-
25pcs/box
-
Storage:
-
4-30°C
-
Method:
-
Immunochromatography
-
Sensitivity:
-
99.6%
-
Brand:
-
MR
Quick Details
-
Warranty:
-
2 years
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Shandong, China
-
Brand Name:
-
MR
-
Model Number:
-
combo-WSP-S
-
Quality Certification:
-
CE
-
Safety standard:
-
ISO14385
-
Product name:
-
HIV HCV HBsAg TP combo cassette
-
Format:
-
cassette
-
Dimension:
-
3.0mm
-
Specimen:
-
whole blood/serum/plasma
-
Package:
-
25pcs/box
-
Storage:
-
4-30°C
-
Method:
-
Immunochromatography
-
Sensitivity:
-
99.6%
-
Brand:
-
MR
Product Description


Specification
Product Name
Human Immunodeficiency Virus Antibody HIV 1/2 Rapid Test Kit (Immunochromatography)
Intended Use
The test is based on the principle of sandwich immunoassay for a qualitative detection of antibody
to human immunodeficiency virus HIV-1/HIV-2 in human serum or plasma.
Principle
Used gene recombination HIV1 and HIV2 mixed antigen P24, gp120, gp41, gp36 and Rab MAb HIV
antibody coated on nitrocellulose as test line and control line. And meanwhile gold colloid
recombination HIV mixed antigen P24, gp120, gp41, gp36, used double antigen sandwich GIA to
detect antibody to HIV-1/HIV-2 in human serum or plasma.
Main Components
Sample pad, colloidal gold marked pad, nitrocellulose membrane, absorbent paper and PVC board.
Storage and Expiry
Store as packaged in the sealed pouch at 4-30°C, avoid hot and sunshine, dry place, valid for 24
months. DO NOT FREEZE. Some protective measures should be taken in hot summer and cold winter
to avoid high temperature or freeze-thaw.
Precaution
1. Operate according to the infectious disease laboratory procedures.
2. If the aluminum foil bag is broken, the kit is affected with damp, cannot be used.
3. Please use the kit in its expiration. Over the test time, the results are invalid.
4. Hyperlipidemia and jaundice samples have no effect to the detection results.
5.Slight hemolysis samples have no effect to the detection results, but serious hemolysis samples
can produce background, impact the observation of test line (T), suggest using other test method.
6. Do not use other kinds of quality control sample to test the reagent. Components of different
batches cannot be exchanged for use to avoid erroneous results.
Manufacturing procedure
Packing & Delivery

carton
Hot Searches