30A SD Top Quality Inner Ear Implantable Surgical Hearing Loss Audiology Healthcare Electrode Medical Nucleus Cochlear Implant
-
Origin:
-
China
-
Payment:
-
T/T Other
-
Export Port:
-
Shenzhen (471401)
-
Model NO:
-
30A SD



- Product Details
- {{item.text}}
Quick Details
Quick Details
About Us
Founded in 2006, headquarter in Hangzhou, China and R&D center in Califonia U.S., Nurotron is dedicated to the rehabilitation of people with disabilities.
Nurotron manufactures neural electrical stimulation therapy and rehabilitation products including:
- cochlear implant system,
- auditory brainstem implantation system,
- artificial vision system,
- deep brain stimulation system,
- hemiplegia stimulation system and etc.
Product Application Fields: The WHO estimates that about 5% of the world's population suffers from disabling hearing loss, affecting their health and quality of life.
Nurotron products are available in 20+ countries.
20000+ users from worldwide have felt the life changes with Nurotron products.





Production Process

Products Description
The raw materials of the implants are all from the United States, comply with FDA standards, have good reliability and biocompatibility, and reduce risk of infection.
Nurotron's implants can resistant against external impact up to 2.5 joules, and helium leakage standards are 50 times stricter than international standards.
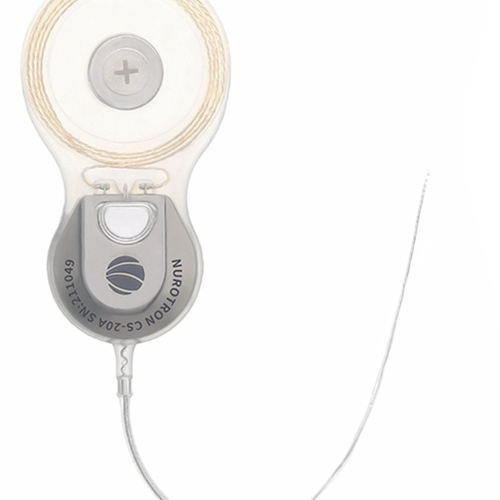
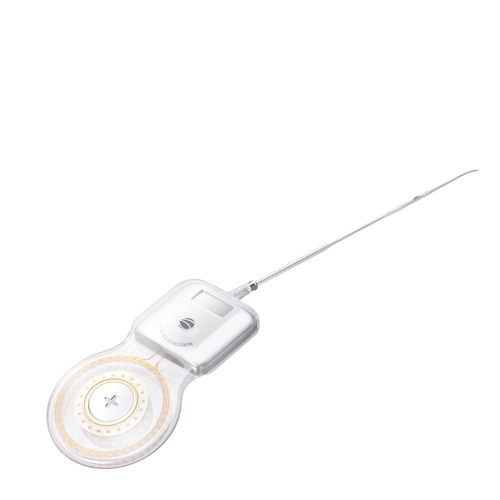
Slim implant body that provides a natural and low-profile appearance designed to minimize need for drilling.
Implant is approved for 1.5T and 3.0T with magnet in place. Removable magnet to reduce artifact, if required.
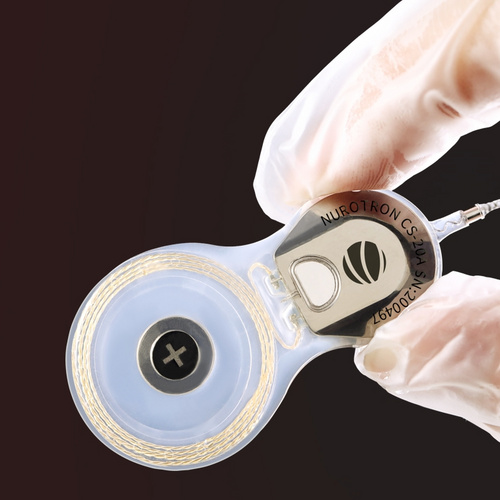
CS-30A has four types of electrode arrays, standard electrode, slim short electrode, slim medium electrode and slim long electrode, with lengths of 16.0mm, 20.5mm and 24.0mm respectively. Different sizes and lengths of electrode arrays provide customization for various types of cochleas.
Except for the TS electrode, which has 22 channels, the other electrodes have 24 channels. More channels can help recipients closest to natural hearing.
The standard electrode has a apical end diameter of 0.5mm and a basal end diameter of 0.9mm. The apical end diameter of the slim electrode is 0.3mm and basal end diameter is 0.8mm. It is moderately soft and hard and is indicated for round window, extended round window and cochleostomy surgical approaches. This is designed by Professor Stephen J Rebscher, the world's top electrode expert. This design not only minimize the occurrence of vertical deviation into the scala media or scala vestibuli, it can also reduce damage to the fine structure of the cochlea and preserve the residual hearing of the recipient.
The implant is available in monopolar, bipolar or common ground stimulation modes, designed for flexible programming options.
Telemetry functions available with the implant include NRM, ESRT, ABR, CEP and intraoperative NRM.
Certification






FAQ
Q: Can I have an MRI after having a cochlear implant?
Answer: Yes! All Nurotron implants can also undergo standard MRIs of 1.5 Tesla. When having an MRI with any Nurotron cochlear implant, please contact the after-sales personnel of Nurotron Company, and we will inform you of the relevant precautions and protective measures.
Q: Can I undergo X-ray examination?
Answer: X-ray as a medical imaging examination will not cause harm to the implant, and the external parts should be removed.
Q: Does the cochlear implant need to be powered off when flying?
Answer: When flying, the speech processor is similar to a computer and has a built-in radio frequency transmitter and needs to be turned off when required. You should inform airline staff about your hearing impairment so that they can remind you of relevant safety measures.
Question: Will wearing a cochlear implant cause an alarm when entering or exiting the supermarket security checkpoint?
A: Electronic item monitors (such as supermarkets). Some cochlear implant recipients may experience sound distortion when passing through or approaching these devices. To avoid this, the processor can be turned off when approaching such devices, despite the implant. It is unlikely that the patient will cause an alarm, but the recipient's identification card should be carried for explanation.
Question: Will the implant cause an alarm when going through airport security?
Answer: Metal detectors (such as airports) will cause strong magnetic fields. If the recipient passes through this system, the material of the implant will cause the metal detector to alarm. The recipient should carry the cochlear implant ID card with him.
Q: Will induction cookers affect the use of cochlear implants?
Answer: The induction cooker uses electromagnetic fields to transmit energy. As long as the recipient is at an appropriate distance, the energy will not be transmitted to the recipient.
Q: Can implant recipients receive electroconvulsive therapy?
Answer: No, electroconvulsive therapy can cause damage to the implant or surrounding tissue.
Q: Can implant recipients receive diathermy?
Answer: No, a large amount of current may be induced into the electrode and cause tissue damage or implant damage. Medical diathermy using ultrasound may be used in areas below the head and neck.