- Product Details
- {{item.text}}
Quick Details
-
CaO Content (%):
-
< 2.5
-
CrO Content (%):
-
0.1
-
SiC Content (%):
-
0.1
-
Place of Origin:
-
Pakistan, Pakistan
-
Model Number:
-
MGO-A001
-
Brand Name:
-
Patel Corporation
-
Color:
-
White
-
Size:
-
lumps 0-100 mm
-
Application:
-
Ceramic, chemical, fertilizer, refractory industries
-
Product name:
-
Magnesium Carbonate, Magnesite
-
Usage:
-
Industrial Usage
-
Sample:
-
Freely Provided
-
Package:
-
25kg/50kg/1MT Bag
-
Type:
-
Ceramic Raw Materials
-
Certificate:
-
S.G.S/B.V/Lab testing report
Quick Details
-
SiO2 Content (%):
-
< 1
-
Al2O3 Content (%):
-
< 1
-
MgO Content (%):
-
>44
-
CaO Content (%):
-
< 2.5
-
CrO Content (%):
-
0.1
-
SiC Content (%):
-
0.1
-
Place of Origin:
-
Pakistan, Pakistan
-
Model Number:
-
MGO-A001
-
Brand Name:
-
Patel Corporation
-
Color:
-
White
-
Size:
-
lumps 0-100 mm
-
Application:
-
Ceramic, chemical, fertilizer, refractory industries
-
Product name:
-
Magnesium Carbonate, Magnesite
-
Usage:
-
Industrial Usage
-
Sample:
-
Freely Provided
-
Package:
-
25kg/50kg/1MT Bag
-
Type:
-
Ceramic Raw Materials
-
Certificate:
-
S.G.S/B.V/Lab testing report
Product Description
MgO: 42 - 45 %
Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, which in the form of a mineral is known as periclase. Large quantities of magnesite are burnt to make magnesium oxide: an important refractory material used as a lining in blast furnaces, kilns, and incinerators. Calcination temperatures determine the reactivity of resulting oxide products and the classifications of light burnt and dead burnt refer to the surface area and resulting reactivity of the product, typically as determined by an industry metric of the iodine number. 'Light burnt' product generally refers to calcination commencing at 450 °C and proceeding to an upper limit of 900 °C - which results in good surface area and reactivity. Above 900 °C, the material loses its reactive crystalline structure and reverts to the chemically inert 'dead-burnt' product- which is preferred for use in refractory materials such as furnace linings.
Magnesite can also be used as a binder in flooring material. Furthermore, it is being used as a catalyst and filler in the
production of synthetic rubber and the preparation of magnesium chemicals and fertilizers.
Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, which in the form of a mineral is known as periclase. Large quantities of magnesite are burnt to make magnesium oxide: an important refractory material used as a lining in blast furnaces, kilns, and incinerators. Calcination temperatures determine the reactivity of resulting oxide products and the classifications of light burnt and dead burnt refer to the surface area and resulting reactivity of the product, typically as determined by an industry metric of the iodine number. 'Light burnt' product generally refers to calcination commencing at 450 °C and proceeding to an upper limit of 900 °C - which results in good surface area and reactivity. Above 900 °C, the material loses its reactive crystalline structure and reverts to the chemically inert 'dead-burnt' product- which is preferred for use in refractory materials such as furnace linings.
Magnesite can also be used as a binder in flooring material. Furthermore, it is being used as a catalyst and filler in the
production of synthetic rubber and the preparation of magnesium chemicals and fertilizers.

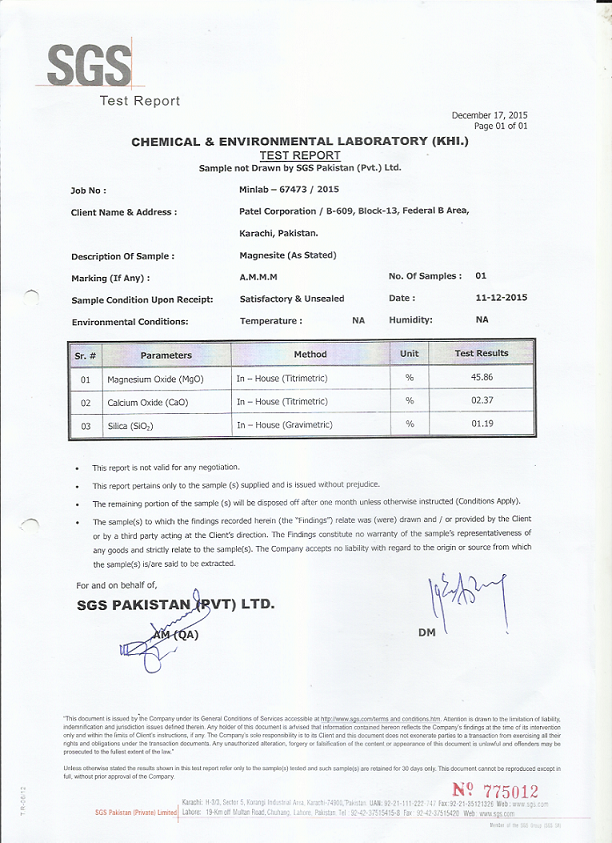

Specification
|
MgO
|
42-45%
|
|
color
|
white
|
|
SiO2
|
<2
|
|
CaO
|
<3
|
Packing & Delivery

To better ensure the safety of your goods, professional, environmentally friendly, convenient, and efficient packaging services will be provided.
Hot Searches













