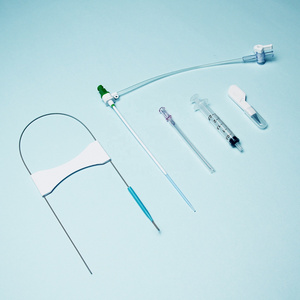
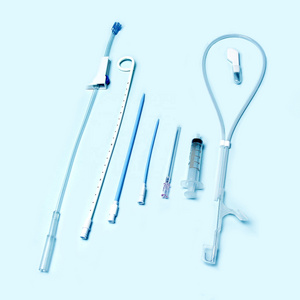
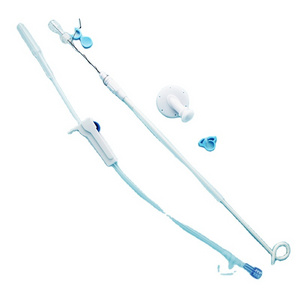
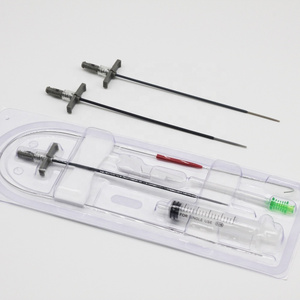
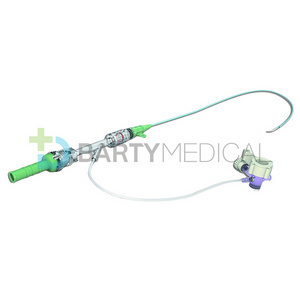
Clinically, Percutaneous Nephrostomy Set is mainly used in patients with kidney stones or
hydronephrosis. It is used for percutaneous renal puncture expansion and channel
establishment for drainage and placement of endoscopic surgical instruments. It
is sterilized by ethylene oxide, the shelf life is 3 years.
- Product Details
- {{item.text}}
Quick Details
-
Size:
-
12F
-
Product name:
-
Tianck Medical percutaneous nephrostomy set
-
Application:
-
Surgical Medical
-
Sterile:
-
E.O.Gas
-
MOQ:
-
40
-
Length:
-
18cm
-
Sample:
-
Sample Provided
-
Brand:
-
Tianck
-
Certificate:
-
CE ISO
-
Package:
-
blister package
-
OEM:
-
Welcomed
Quick Details
-
Place of Origin:
-
Guangdong, China
-
Brand Name:
-
Tianck Medical
-
Model Number:
-
with peelable sheath
-
Size:
-
12F
-
Product name:
-
Tianck Medical percutaneous nephrostomy set
-
Application:
-
Surgical Medical
-
Sterile:
-
E.O.Gas
-
MOQ:
-
40
-
Length:
-
18cm
-
Sample:
-
Sample Provided
-
Brand:
-
Tianck
-
Certificate:
-
CE ISO
-
Package:
-
blister package
-
OEM:
-
Welcomed
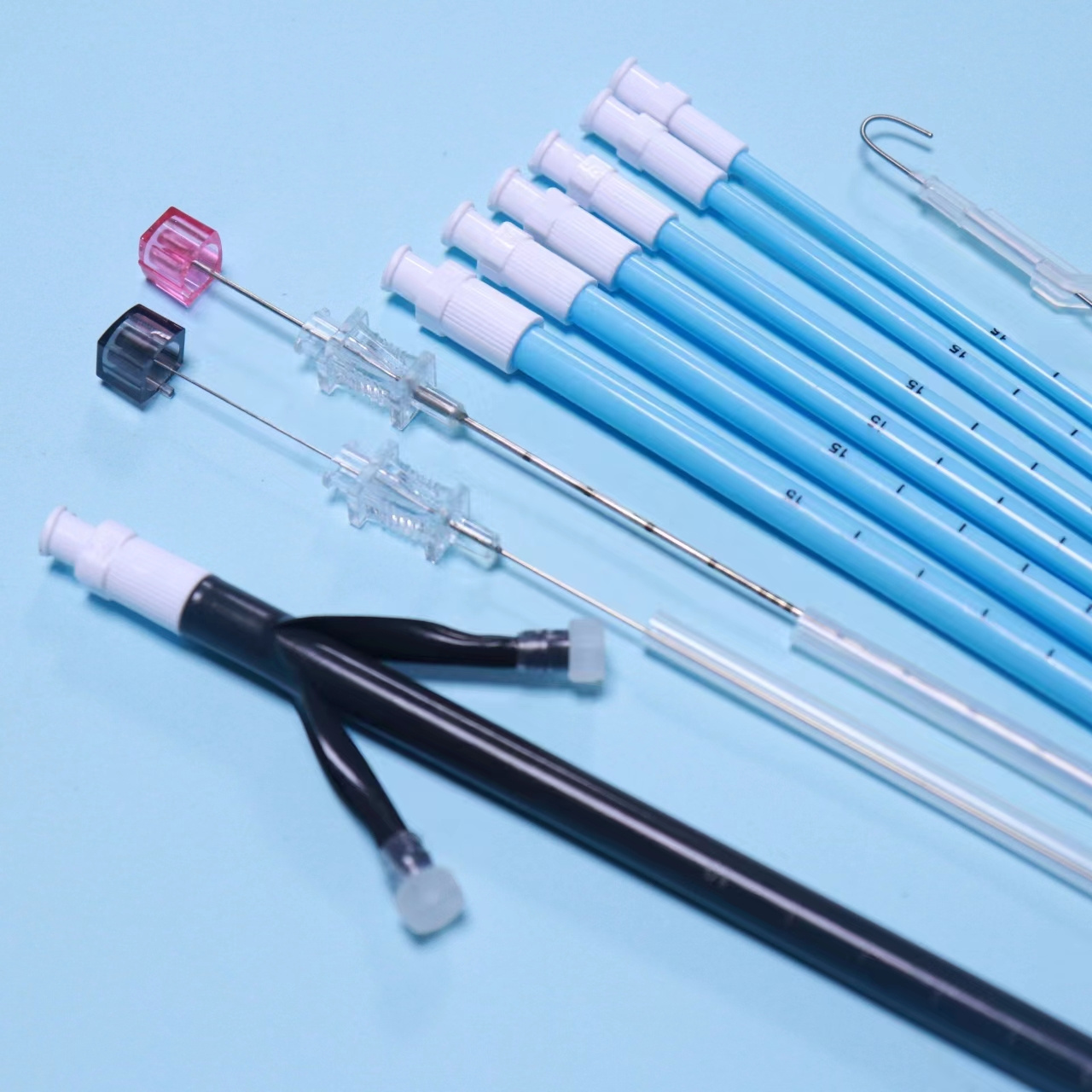
Products Description
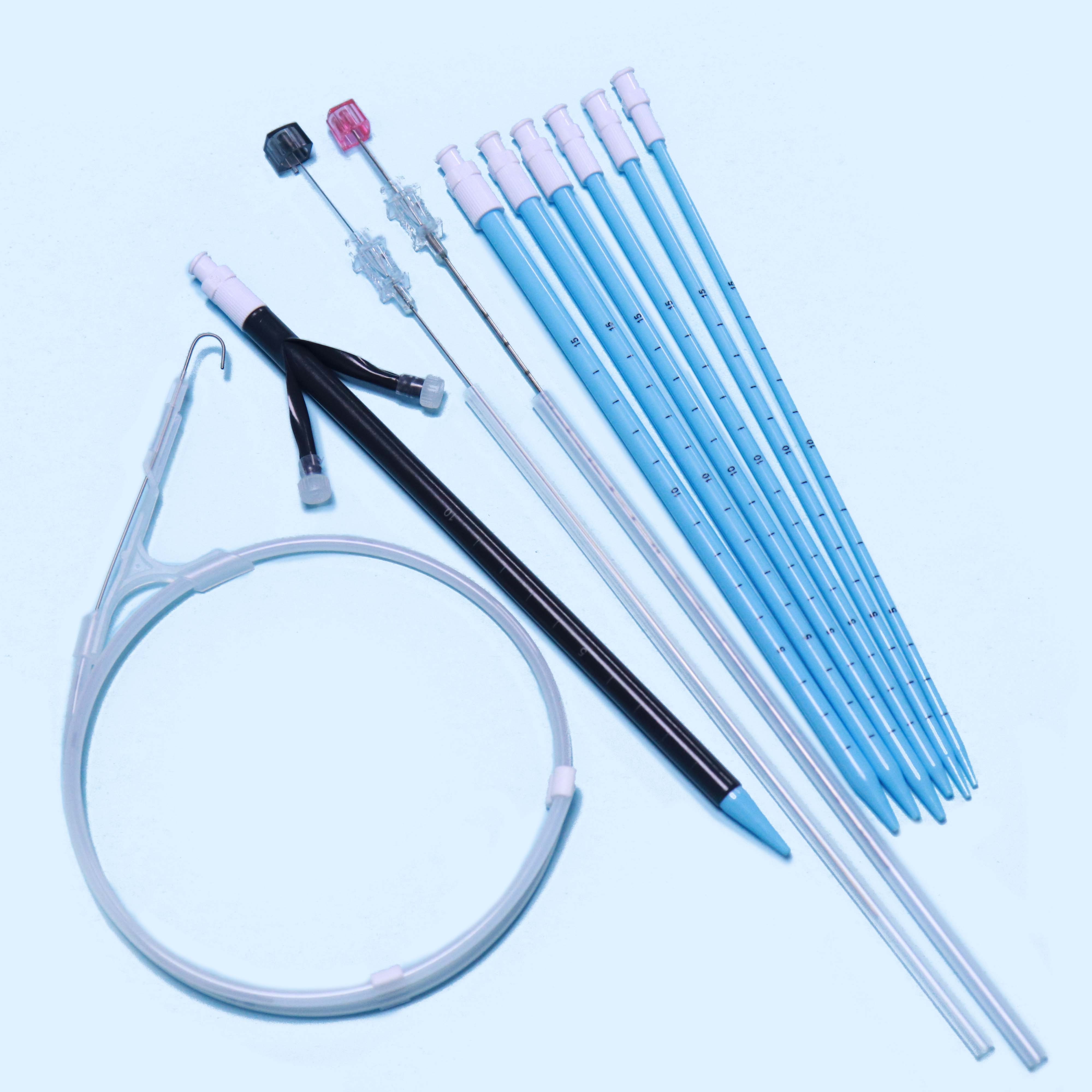
Tianck Medical
Percutaneous Nephrostomy Set
Specification
|
ITEM
|
VALUE
|
|
Product name
|
Tianck Medical Percutaneous Nephrostomy Set
|
|
Place of Origin
|
China
|
|
Brand Name
|
Tianck medical
|
|
Disinfecting Type
|
EOS
|
|
Properties
|
The Basis of Surgical Instruments
|
|
Model
|
12F 14F 16F 18F 20F 22F 18cm
|
|
Stock
|
NO
|
|
Shelf Life
|
3 years
|
|
Quality Certification
|
ce
|
|
Instrument classification
|
Class II
|
|
Safety standard
|
EN 149 -2001+A1-2009
|
|
MOQ
|
40pcs
|
|
Sample
|
Charge
|
1. Intended use
Clinically, Percutaneous Nephrostomy Set is mainly used in patients with kidney stones or hydronephrosis. It is used for percutaneous renal puncture expansion and channel establishment for drainage and placement of endoscopic surgical instruments.
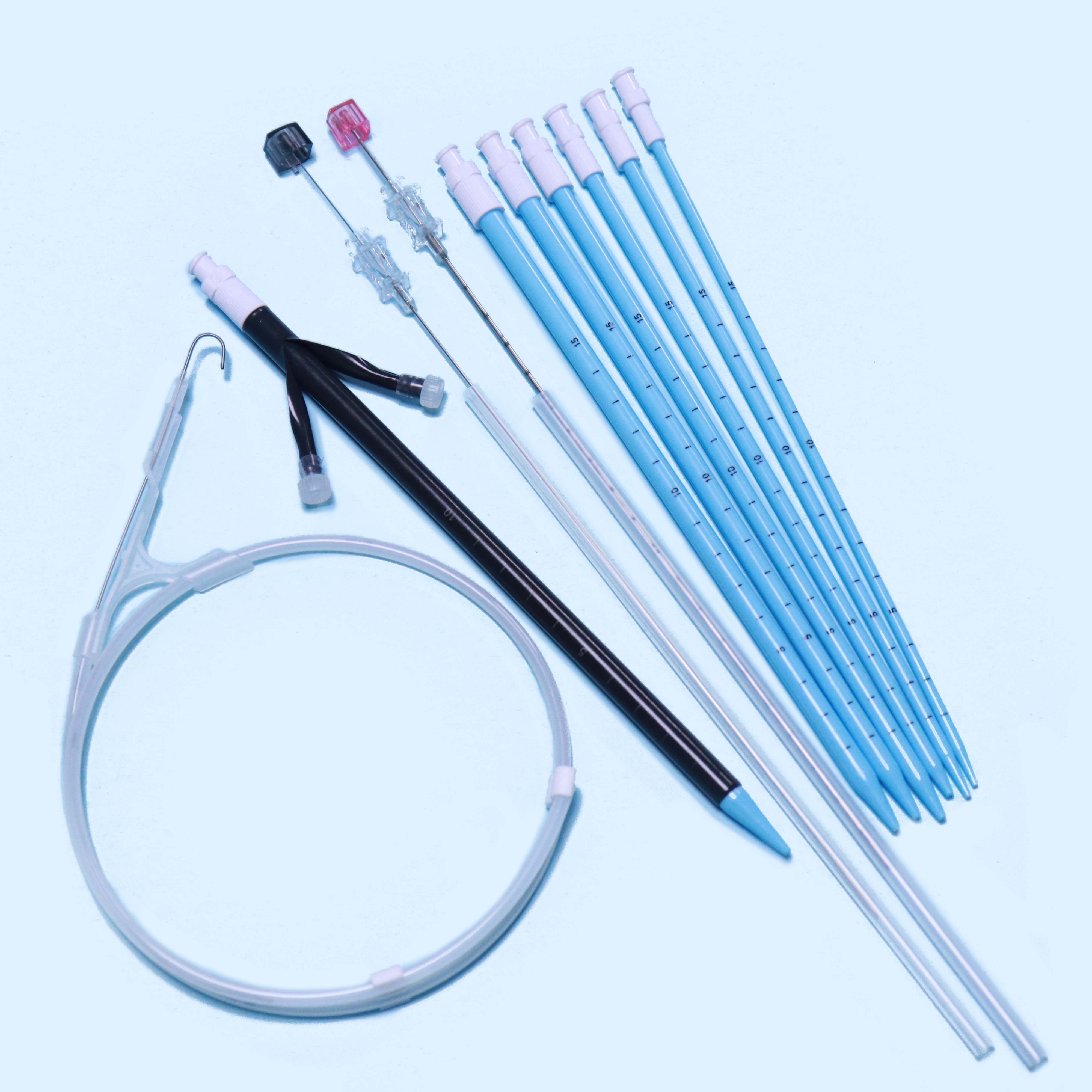
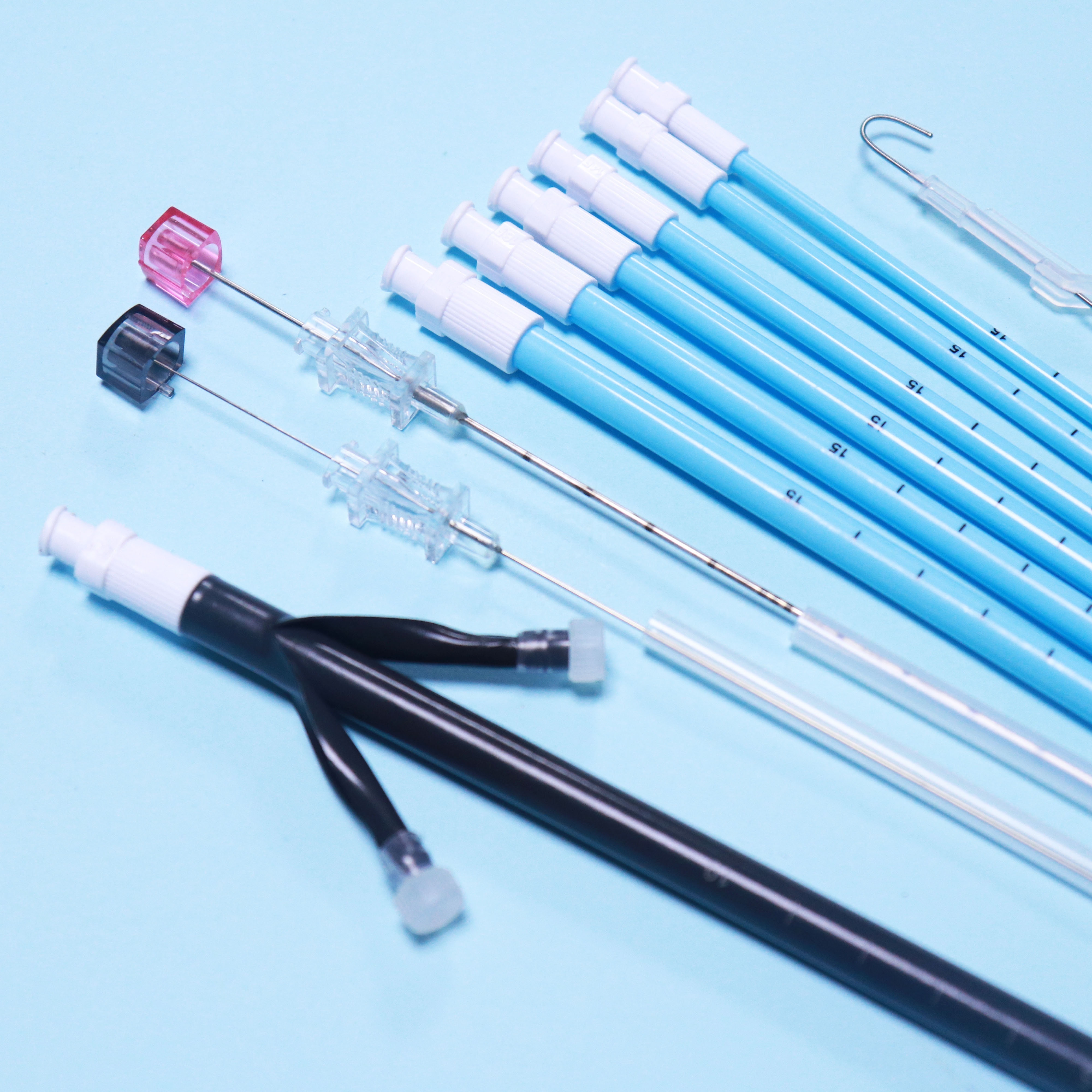
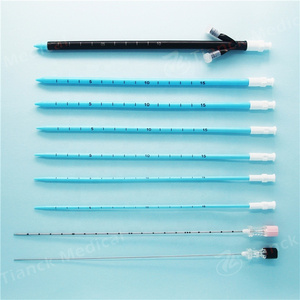
2. Structure and material
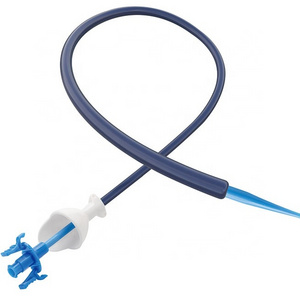
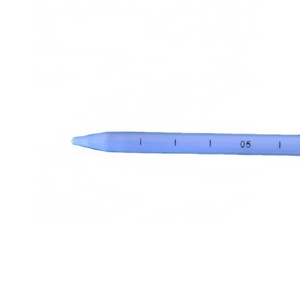
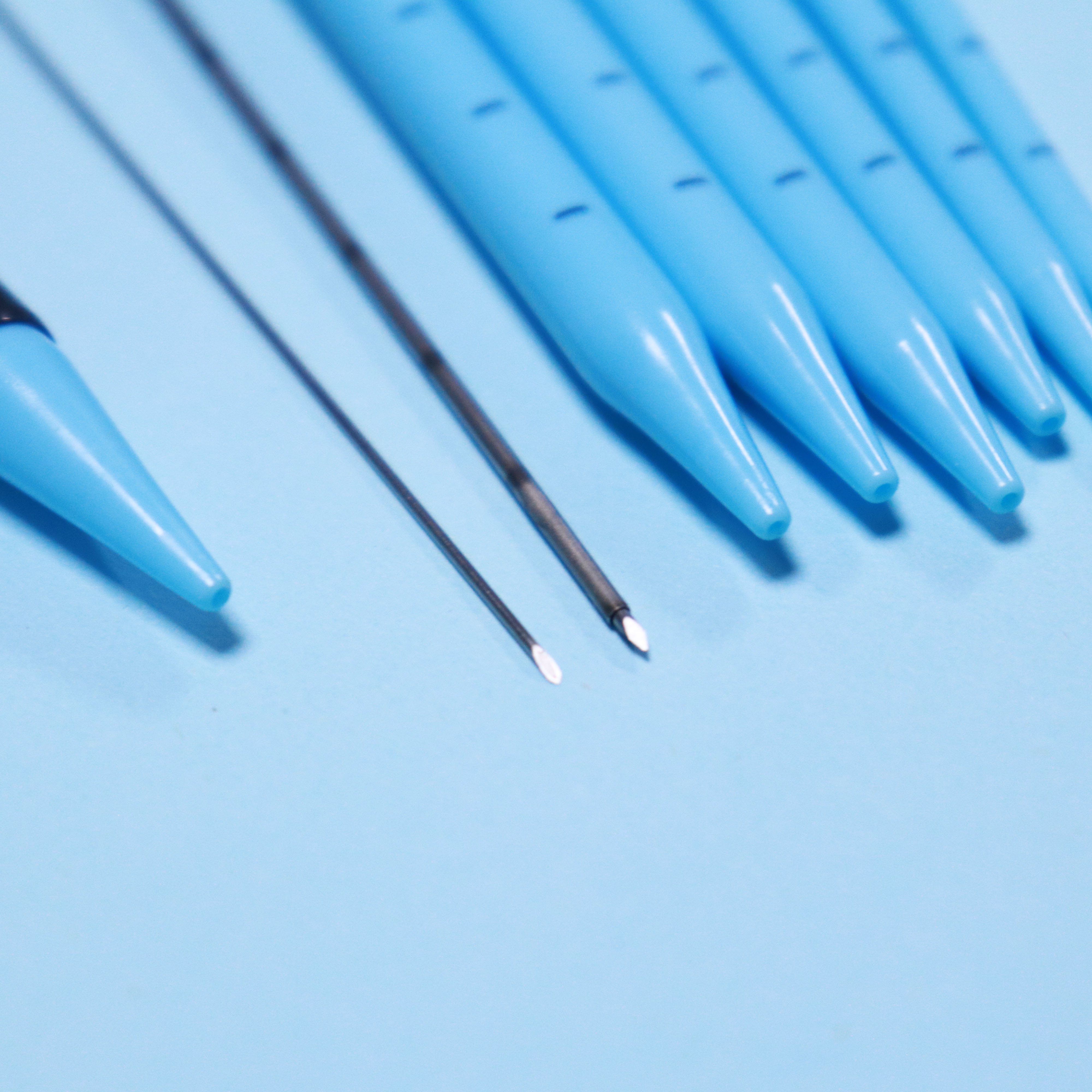
The Percutaneous nephrostomy set consists of peelable sheath, dilator, guidewire and guide needle.
Peelable sheath is made of PTFE; Dilator is made of PE; Guidewire is made of stainless steel and PTFE; Guide needle is made of stainless steel and polycarbonate.
3. Contraindication
None known.
4. Warning
This product is EO sterilized. The package should be checked before using. No use if unsealing, gas leakage or breakage are found.
This product must be used before expiry date.
The product has multiple specifications, select the appropriate specification according to the patient's situation.
The device should be used immediately after opening the package.
The device should be disposed in proper way after use in accordance with local laws or hospital regulations.
The product is single use product, no repeat sterilization or reuse, it may cause cross infection.
This product is EO sterilized. The package should be checked before using. No use if unsealing, gas leakage or breakage are found.
This product must be used before expiry date.
The product has multiple specifications, select the appropriate specification according to the patient's situation.
The device should be used immediately after opening the package.
The device should be disposed in proper way after use in accordance with local laws or hospital regulations.
The product is single use product, no repeat sterilization or reuse, it may cause cross infection.
5. Instruction for use
Select the puncture site for disinfection and anesthesia;
Make a small incision at the puncture site with scalpel;
Insert the puncture needle with needle core at puncture site and reach the collection system, removes the needle core, the puncture is success if there is urine out.
Insert the guide wire through the needle sheath into the kidney collection system, better through the ureter, so that the guide wire is not easy to come out or twist during the expansion of the channel.
After guide wire placement, withdraw the needle sheath;
Start dilation from 8F dilator, insert the dilator along the guide wire. Pull the guide wire
gently and fix it with one hand to keep the guide wire at a certain tension. Push and rotate the dilator to the set depth, there would be urine out;0197
Insert larger dilator in sequence according to the size one by one until it can accommodate the peelable sheath for surgery;
Withdraw dilator and retain the peelable sheath to establish the surgical channel for needed devices;
If the needed devices need long-term indwelling, the peelable sheath can be peeled apart after the operation and withdrawn.
Recommend Products
Hot Searches