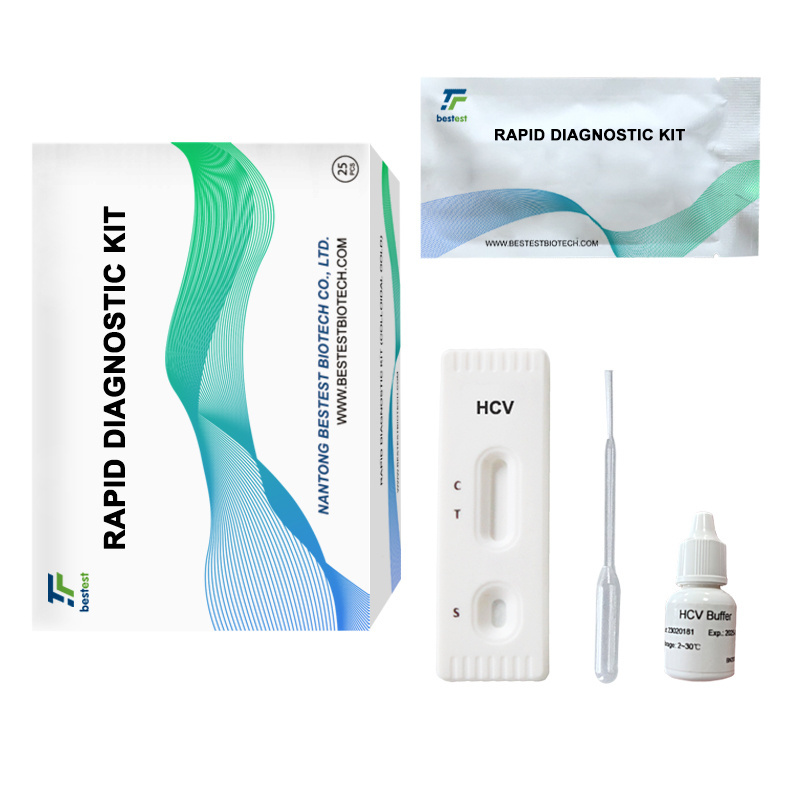
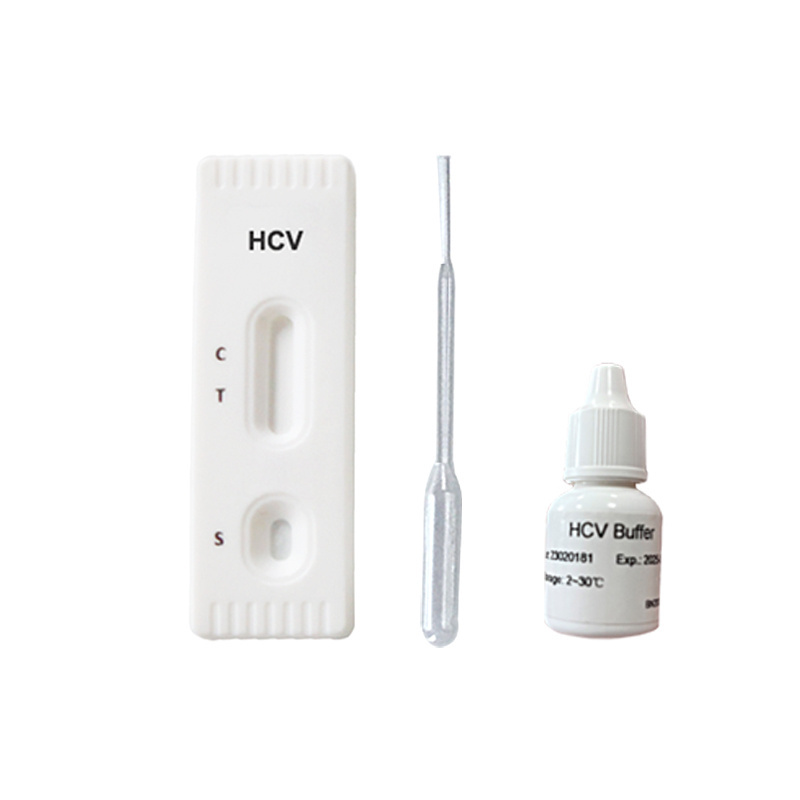
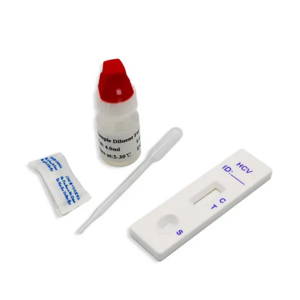
- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
Bestest
-
Model Number:
-
hcv rapid test kits
-
Quality Certification:
-
iso
-
specimen:
-
whole blood/serum/plasma
-
accuracy:
-
over 99%
Quick Details
-
Warranty:
-
2 years
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Jiangsu, China
-
Brand Name:
-
Bestest
-
Model Number:
-
hcv rapid test kits
-
Quality Certification:
-
iso
-
specimen:
-
whole blood/serum/plasma
-
accuracy:
-
over 99%
Products Description
A rapid test for the qualitative detection of antibodies to Hepatitis C Virus in whole blood,
serum or plasma.
For professional in vitro diagnostic use only.
【INTENDED USE】
The HCV Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic
immunoassay for the qualitative detection of antibody to Hepatitis C Virus in whole blood,
serum or plasma.
【SUMMARY】
Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA Virus.
HCV is now known to be the major cause of parenterally transmitted non-A, non-B hepatitis.
Antibody to HCV is found in over 80% of patients with well-documented non-A, non-B hepatitis.
Conventional methods fail to isolate the virus in cell culture or visualize it by electron
microscope. Cloning the viral genome has made it possible to develop serologic assays that
use recombinant antigens.1, 2 Compared to the first generation HCV EIAs using single
recombinant antigen, multiple antigens using recombinant protein and/or synthetic peptides
have been added in new serologic tests to avoid nonspecific cross-reactivity and to increase
the sensitivity of the HCV antibody tests.3,4
The HCV Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid test to qualitatively
detect the presence of antibody to HCV in a whole blood, serum or plasma specimen. The test
utilizes colloid gold conjugate and recombinant HCV proteins to selectively detect antibody to
HCV in whole blood, serum or plasma. The recombinant HCV proteins used in the test kit are
encoded by the genes for both structural (nucleocapsid) and non-structural proteins.
serum or plasma.
For professional in vitro diagnostic use only.
【INTENDED USE】
The HCV Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic
immunoassay for the qualitative detection of antibody to Hepatitis C Virus in whole blood,
serum or plasma.
【SUMMARY】
Hepatitis C Virus (HCV) is a small, enveloped, positive-sense, single-stranded RNA Virus.
HCV is now known to be the major cause of parenterally transmitted non-A, non-B hepatitis.
Antibody to HCV is found in over 80% of patients with well-documented non-A, non-B hepatitis.
Conventional methods fail to isolate the virus in cell culture or visualize it by electron
microscope. Cloning the viral genome has made it possible to develop serologic assays that
use recombinant antigens.1, 2 Compared to the first generation HCV EIAs using single
recombinant antigen, multiple antigens using recombinant protein and/or synthetic peptides
have been added in new serologic tests to avoid nonspecific cross-reactivity and to increase
the sensitivity of the HCV antibody tests.3,4
The HCV Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid test to qualitatively
detect the presence of antibody to HCV in a whole blood, serum or plasma specimen. The test
utilizes colloid gold conjugate and recombinant HCV proteins to selectively detect antibody to
HCV in whole blood, serum or plasma. The recombinant HCV proteins used in the test kit are
encoded by the genes for both structural (nucleocapsid) and non-structural proteins.
【DIRECTIONS FOR USE】
Allow test cassette, specimen, and/or controls to equilibrate to room temperature (15-30°C)
prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test cassette from the
sealed pouch and use it as soon as possible. Best results will be obtained if the assay is
performed within one hour.
2. Place the cassette on a clean and level surface.
For Serum or Plasma specimen: Hold the dropper vertically and transfer 1 drop of serum
or plasma (approximately 25 L) to the specimen area, then add 2 drops of buffer
(approximately 80 L),and start the timer, see illustration below.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2
drops of whole blood (approximately 50 L) to the specimen area, then add 2 drops of
buffer (approximately 80L), and start the timer. See illustration below.
For Fingerstick Whole Blood specimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50 L of
fingerstick whole blood specimen to the specimen area of test cassette, then add 2
drops of buffer (approximately 80 L) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. The test result should be read at 10 minutes. Do not
interpret the result after 20 minutes.
Allow test cassette, specimen, and/or controls to equilibrate to room temperature (15-30°C)
prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test cassette from the
sealed pouch and use it as soon as possible. Best results will be obtained if the assay is
performed within one hour.
2. Place the cassette on a clean and level surface.
For Serum or Plasma specimen: Hold the dropper vertically and transfer 1 drop of serum
or plasma (approximately 25 L) to the specimen area, then add 2 drops of buffer
(approximately 80 L),and start the timer, see illustration below.
For Venipuncture Whole Blood specimen: Hold the dropper vertically and transfer 2
drops of whole blood (approximately 50 L) to the specimen area, then add 2 drops of
buffer (approximately 80L), and start the timer. See illustration below.
For Fingerstick Whole Blood specimen:
To use a capillary tube: Fill the capillary tube and transfer approximately 50 L of
fingerstick whole blood specimen to the specimen area of test cassette, then add 2
drops of buffer (approximately 80 L) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. The test result should be read at 10 minutes. Do not
interpret the result after 20 minutes.
|
Product Name
|
ready to ship hcv devices rapid test hcv device kit hepatitis
|
|
Brand Name
|
Bestest, OEM accepted
|
|
Methodology
|
Colloidal gold immune chromatographic assay
|
|
Format
|
Strip / Cassette (midstream for HCG,LH,FSH; cup for multi-drag Test)
|
|
Size
|
Strip: 2.5mm/3mm/4mm/5mm
|
|
|
Cassette : 3mm/4mm
|
|
|
Midstream: 3mm/5mm/5.5mm/6mm
|
|
Accuracy
|
More than 99%
|
|
Reaction
|
1-5 minutes
|
|
Time Reading time
|
3-5 minutes
|
|
Shelf Life
|
2years / 24 months
|
|
Range of application
|
All levels of medical units and home self-test
|
|
Bestest One Step Fertility & Eugenic Test Products Profile
|
|
|
|
|
||||
|
No.
|
Product
|
Specimen
|
Type
|
Sensitivity
|
||||
|
BF2001
|
Pregnancy HCG Test Kit
|
Urine/Serum
|
strip/cassette/midstream
|
25mIU/ml
|
||||
|
BF2002
|
Digital Pregnancy Test Kit
|
Urine
|
midstream
|
25mIU/ml
|
||||
|
BF2003
|
Ovulation (LH) Test Kit
|
Urine
|
strip/cassette/midstream
|
25mIU/ml
|
||||
|
BF2004
|
FSH(Follicle-stimulating Hormone)
|
Urine
|
strip/cassette/midstream
|
25mIU/ml
|
||||
|
BF2005
|
fFN(Fetal Fibronectin)
|
cervical mucus
|
strip/cassette
|
25ng/ml
|
||||
|
BF2006
|
Sperm-density
|
Semen
|
cassette
|
N/A
|
||||
|
BF2007
|
HSV I lgG/lgM
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2008
|
HSV II lgG/lgM
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2009
|
HSV 1+2 lgG/lgM
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2010
|
TOXO IgG/IgM
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2011
|
RV IgG/IgM
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2012
|
CMV IgG/IgM
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2013
|
TORCH IgM combo-5
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
|
BF2014
|
TORCH IgG/IgM combo-5
|
whole blood/ serum/plasma
|
cassette
|
N/A
|
||||
Specification
1.Facilitates patient treatment decisions quickly
2.Simple, time-saving procedure
3.All necessary reagents provided & no equipment needed.
4.High sensitivity and specificity
5.Shelf life: 24 months
6.Storage: 2-30°C
Precautions
1. For professional in vitro diagnostic use only. Do not use after the expiration date.
2. The test should remain in the sealed pouch or closed canister until ready to use.
3. Do not eat, drink or smoke in the area where the specimens or kits are handled.
4. Do not use the test if the pouch is damaged.
5. All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
6. Wear protective clothing such as laboratory coats, disposable gloves or eye protection when specimens are being tested.
7. The used test should be discarded according to local regulations.
8. Humidity and temperature can adversely affect results.
Test Principle and technical indicators
Principle:
The kit for sperm count detection (Colorimetric method) used the inert glass fiber membrane with high water absorption and the pore size of less than 0.5um to filter the semen, and sperm cells will be trapped on surface of the first layer of the membrane,and we use the staining fluid that can quickly dye sperm cells, the darker of the color, the more of sperm count on the filtermembrane, and the count of sperm in the semen will be higher. Through comparing with the reference color (15million/ml specifiedby WHO) of well B on the test board, we know that if color of the test well A is lighter than color of the color card, the spermcount is positive, and if color of the test well A is darker than the color card, the sperm count is negative.
Indicators of product technical characteristics:
This kit can only be used as an in vitro diagnostic test using human semen specimen and cannot be used with specimens of other bodily fluids The kit shall be stored at room temperature,avoiding areas of excess moisture If the foil packaging is damaged or has been opened,please do not use.
This test kit is a preliminary test and a repeated negative result should be discussed with your doctor or medical professional.
The kit must not be frozen or used after the expiry date printed on the outer foil.
This test kit is a preliminary test and a repeated negative result should be discussed with your doctor or medical professional.
The kit must not be frozen or used after the expiry date printed on the outer foil.
Packing&Delivery
|
Packing instructions:
|
|
|
||
|
Type
|
Foil pouch Size(cm)
|
Package
|
||
|
strip
|
11.5*5.5
|
100T/valve bag---5000T/carton
|
||
|
|
11.5*5.5
|
100T/box ---5000T/carton
|
||
|
|
11.5*5.5
|
100T/valve bag---10000T/carton
|
||
|
|
11.5*5.5
|
50T/box ---10000T/carton
|
||
|
cassette
|
12*6
|
40T/box ---2000T/carton
|
||
|
|
12*6
|
50T/box ---2500T/carton
|
||
|
|
12*6
|
25T/box ---2000T/carton
|
||
|
|
12*6
|
100T/valve bag---2000T/carton
|
||
|
midstream
|
18.8*5.5
|
1T/box ---500T/carton
|
||
|
|
18.8*5.5
|
20T/box ---1000T/carton
|
||
|
|
18.8*5.5
|
50T/valve bag---2000T/carton
|
||
|
Delivery:
|
|
|
||
|
Quantity Below 5000 pieces, delivery in 5 days after receive deposit
|
|
|
||
Hot Searches