- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
tianzuo
-
Model Number:
-
TW8111/TW8121/TW8131/TW8141
-
Shelf Life:
-
5 years
-
Type:
-
First-Aid Devices
-
Name:
-
silicone manual resuscitator
-
Package:
-
plastic case/paper box/poly bag
-
Function:
-
pulmonary resuscitation
-
Item No.:
-
TW8111/TW8121/TW8131
-
Certificate:
-
CE/ISO
-
Color:
-
neutral, customize accepted
-
Feature:
-
100% latex free
-
OEM:
-
Avalibale
Quick Details
-
Warranty:
-
5 years
-
After-sale Service:
-
Free spare parts
-
Place of Origin:
-
Fujian, China
-
Brand Name:
-
tianzuo
-
Model Number:
-
TW8111/TW8121/TW8131/TW8141
-
Shelf Life:
-
5 years
-
Type:
-
First-Aid Devices
-
Name:
-
silicone manual resuscitator
-
Package:
-
plastic case/paper box/poly bag
-
Function:
-
pulmonary resuscitation
-
Item No.:
-
TW8111/TW8121/TW8131
-
Certificate:
-
CE/ISO
-
Color:
-
neutral, customize accepted
-
Feature:
-
100% latex free
-
OEM:
-
Avalibale
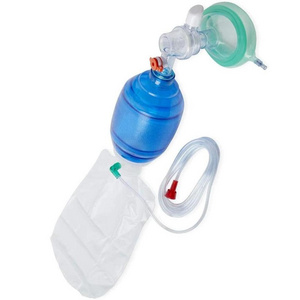
Ambulance silicone manual resuscitator
Welcome to check our products:Manual Resuscitator,Face Mask,Air Splints & Extrication Collar,Anesthesia Items and so on,details as below:
Intended Use
Silicone resuscitator is intended for pulmonary resuscitation.
Highlights
Good functioning
Excellent expanding
Perfect perception
Item No.
TW8111-Adult: for adults and children
TW8121-Pediatric: for children and infants
TW8131-Infant: for infants and neonates
TW8141-Neonate: for neonate
Optional Accessories
silicone face mask, oxygen tube,reservoir bag,
PEEP valve, reservoir valve, airways, nose clip, mouth opener
Optional Package
pp case/paper box/poly bag
Speciality
1. Made of pure, clear, durable silicone, 100% latex free
2. All parts can be easily dismounted for cleaning and disinfecting
3. Silicone resuscitator bag and silicone face mask can be autoclaved repeatedly at 134 degree
4. Includes pressure relief valve to minimize the risk of over pressure be required, the pressure relief valve can be blocked
5. Silicone resuscitator conforms to the standard: ISO10651-4:2002, and it also conforms to Council Directive MDD/93/42/ECC concerning Medical Devices
Details