- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
EGENS/OEM
-
Model Number:
-
ID14-01
-
Product name:
-
Malaria Pf Pv test kit
-
Type:
-
Blood Testing Equipments
-
Specimen:
-
whole blood
-
Format:
-
cassette
-
Read Time:
-
15min
-
Accracy:
-
>96.5%
-
Certificate:
-
CE FSC ISO
-
OEM/ODM:
-
Availabe
Quick Details
-
Warranty:
-
3 years
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Jiangsu, China
-
Brand Name:
-
EGENS/OEM
-
Model Number:
-
ID14-01
-
Product name:
-
Malaria Pf Pv test kit
-
Type:
-
Blood Testing Equipments
-
Specimen:
-
whole blood
-
Format:
-
cassette
-
Read Time:
-
15min
-
Accracy:
-
>96.5%
-
Certificate:
-
CE FSC ISO
-
OEM/ODM:
-
Availabe
Products Description

Product details
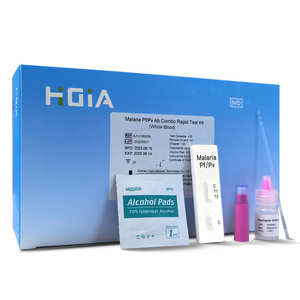
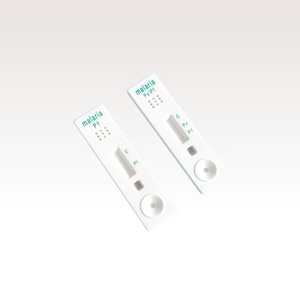
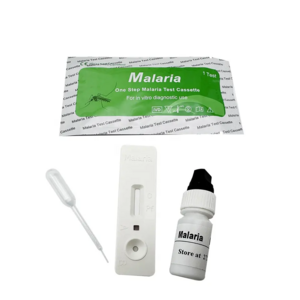
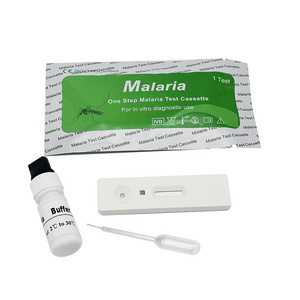
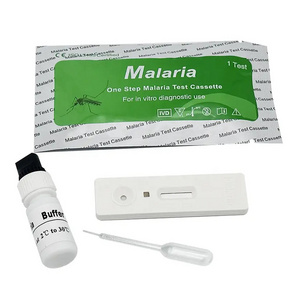
Malaria P.v / P.f Antigen Test Cassette
(Whole Blood)
INTENDED USE
Malaria P.v/P.f Antigen Test is a lateral flow chromatographic immunoassay for the qualitative detection of Malaria P.falciparum specific histidine rich protein-Ⅱ(Pf HRP-Ⅱ) and malaria PLDH specific lactate in whole blood .This assay provides only a preliminary qualitative test result. This device is intended to be used as a screening test and as an aid in the diagnosis of infection with plasmodium. Any reactive specimen with the Malaria P.v/P.f Antigen Test must be confirmed with alternative testing method(s) and clinical findings.
INTRODUCTION
Malaria is a mosquito-borne, hemolytic, febrile illness that infects over 200 million people and kills more than 1 million people per year. It is caused by four species of Plasmodium: P. falciparum, P. vivax, P. ovale and P. malariae. These Plasmodium spp. all infect and destroy human erythrocytes producing chills, fever, anemia and splenomegaly. P. falciparum causes more severe disease than the other Plasmodium species and accounts for most malaria deaths.. Early detection of P.f malaria is of paramount importance due to incidence of cerebral malaria and drug resistance associated with it.
Traditionally, malaria is diagnosed by the demonstration of the organisms on Giemsa stained smears of peripheral blood, and the different species of Plasmodium are distinguished by their appearance in infected erythrocytes. The technique is capable of accurate and reliable diagnosis, but only when performed by skilled microscopists using defined protocols, which presents major obstacles for the remote and poor areas of the world.
The Malaria P.v/P.f Antigen Test is developed for solving the above obstacles. It detects the antibodies generated in blood
specimen in response to the infection of Plasmodium.
Malaria P.v/P.f Antigen Test is a lateral flow chromatographic immunoassay for the qualitative detection of Malaria P.falciparum specific histidine rich protein-Ⅱ(Pf HRP-Ⅱ) and malaria PLDH specific lactate in whole blood .This assay provides only a preliminary qualitative test result. This device is intended to be used as a screening test and as an aid in the diagnosis of infection with plasmodium. Any reactive specimen with the Malaria P.v/P.f Antigen Test must be confirmed with alternative testing method(s) and clinical findings.
INTRODUCTION
Malaria is a mosquito-borne, hemolytic, febrile illness that infects over 200 million people and kills more than 1 million people per year. It is caused by four species of Plasmodium: P. falciparum, P. vivax, P. ovale and P. malariae. These Plasmodium spp. all infect and destroy human erythrocytes producing chills, fever, anemia and splenomegaly. P. falciparum causes more severe disease than the other Plasmodium species and accounts for most malaria deaths.. Early detection of P.f malaria is of paramount importance due to incidence of cerebral malaria and drug resistance associated with it.
Traditionally, malaria is diagnosed by the demonstration of the organisms on Giemsa stained smears of peripheral blood, and the different species of Plasmodium are distinguished by their appearance in infected erythrocytes. The technique is capable of accurate and reliable diagnosis, but only when performed by skilled microscopists using defined protocols, which presents major obstacles for the remote and poor areas of the world.
The Malaria P.v/P.f Antigen Test is developed for solving the above obstacles. It detects the antibodies generated in blood
specimen in response to the infection of Plasmodium.
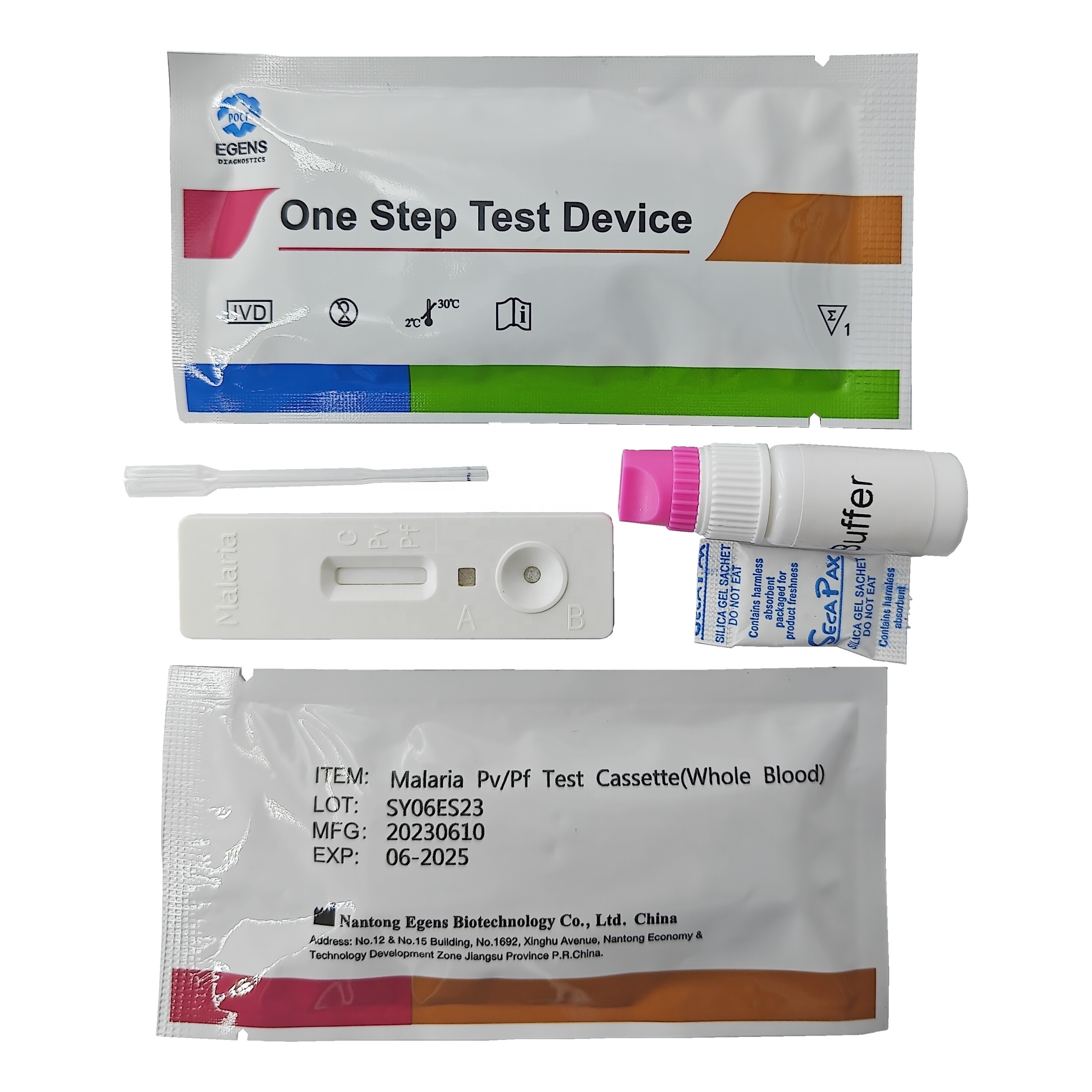
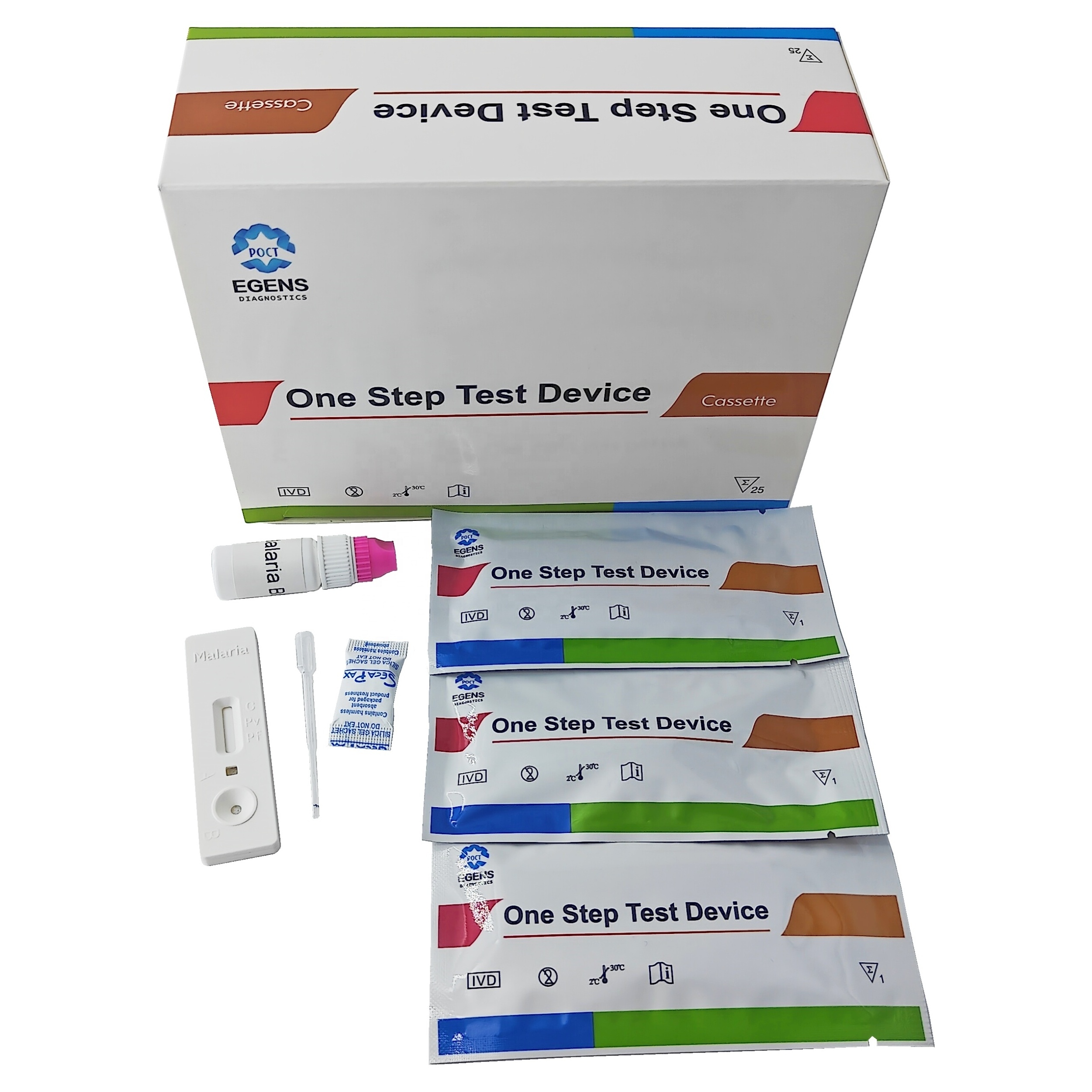
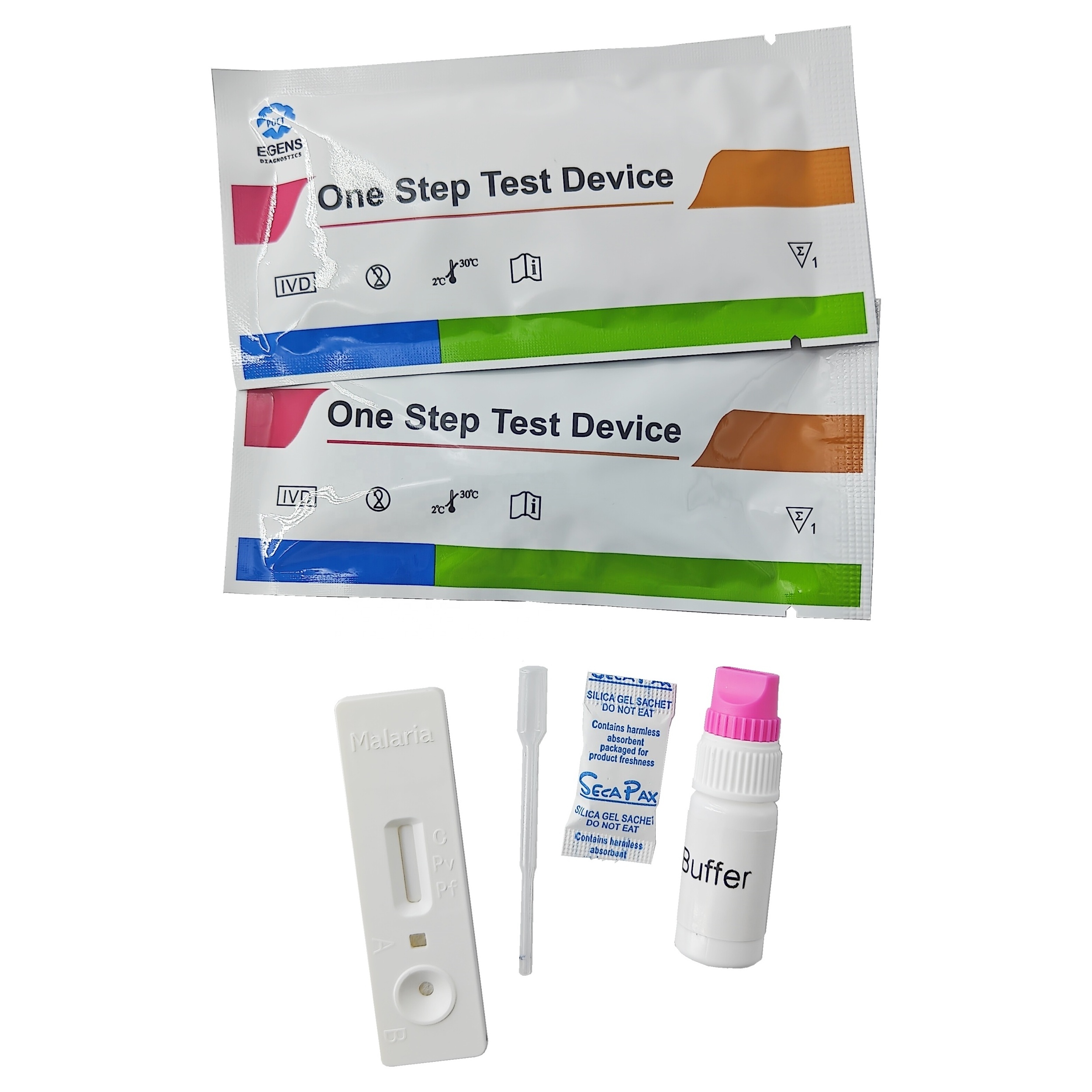
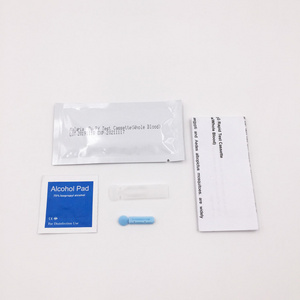
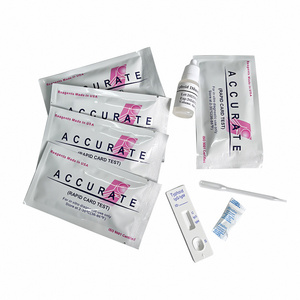
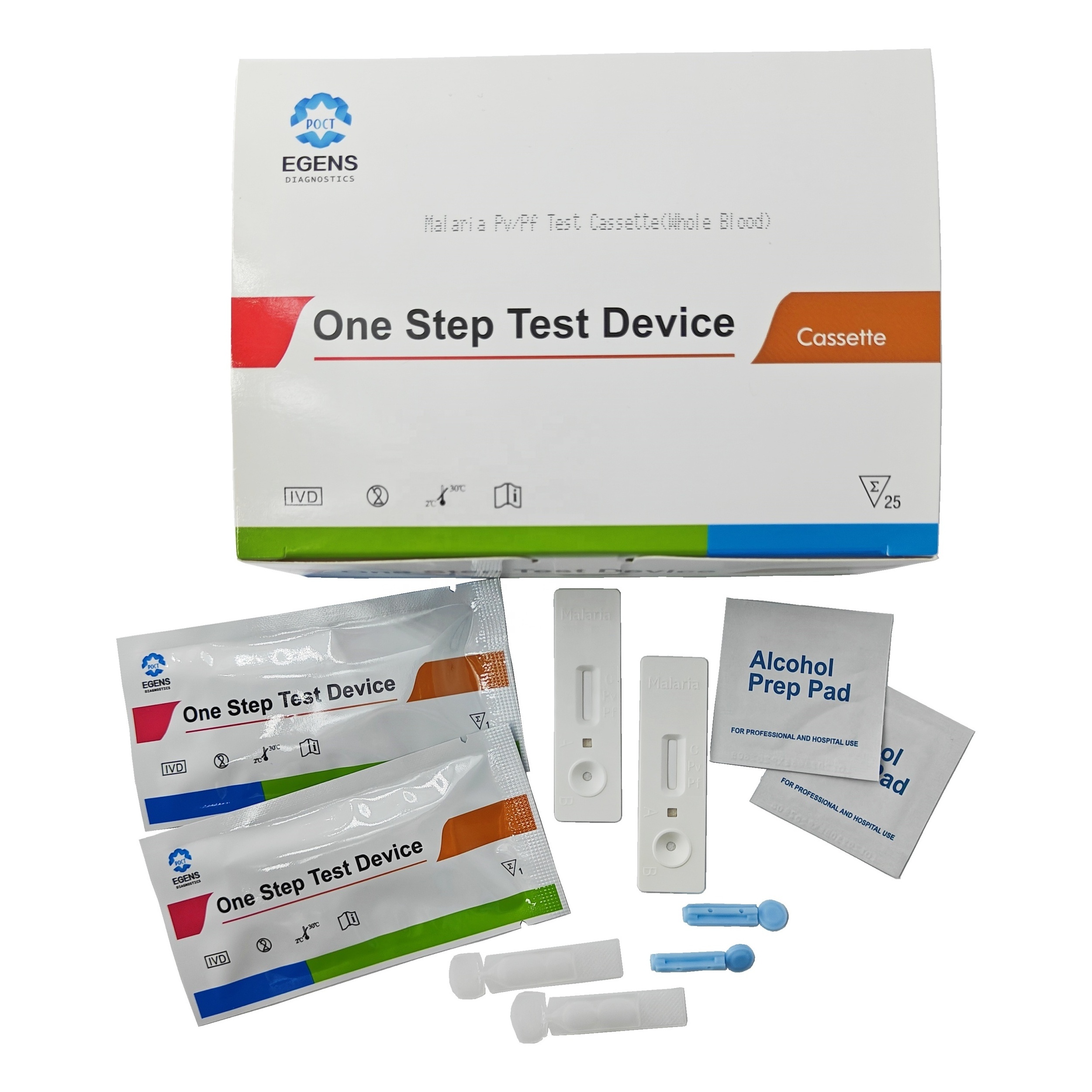
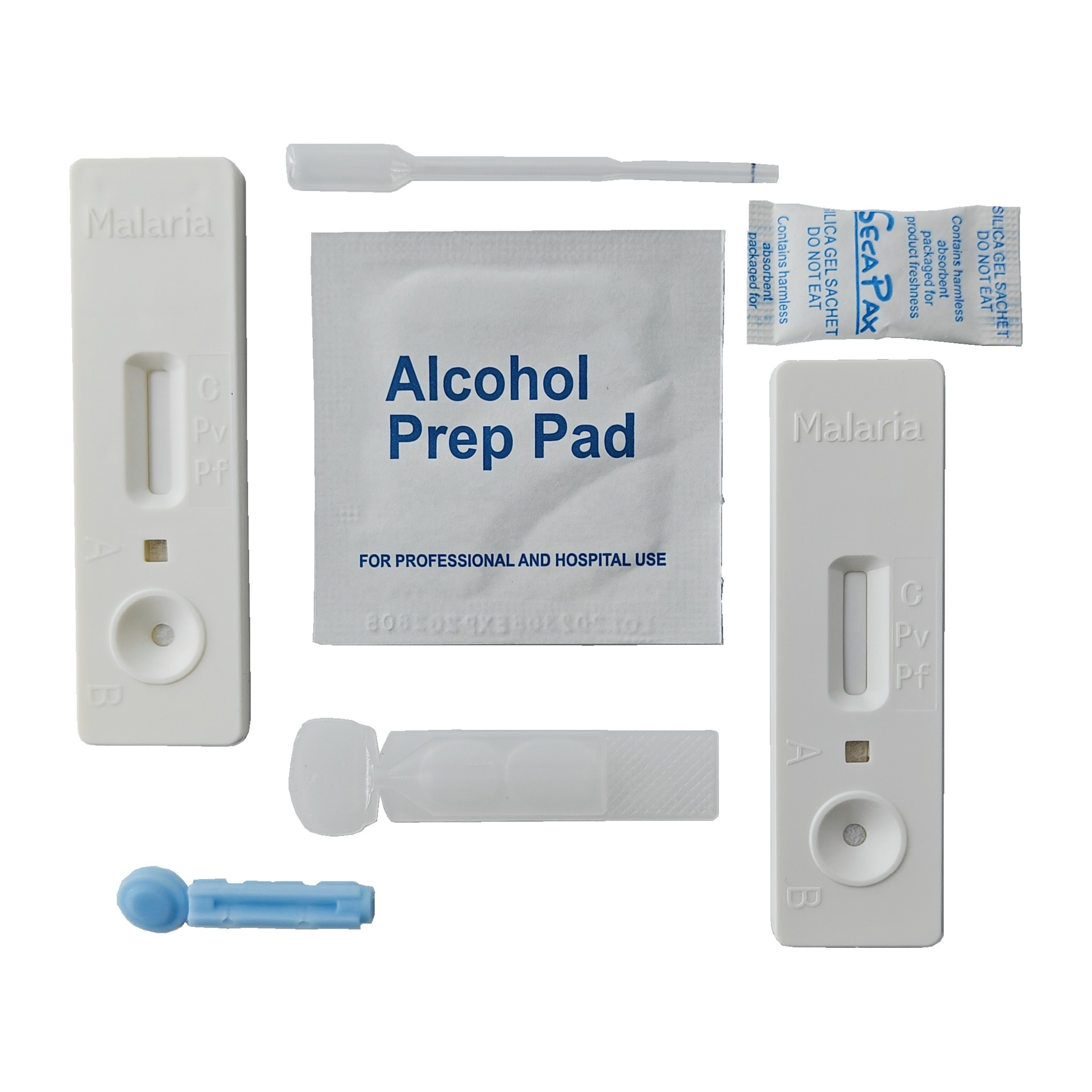
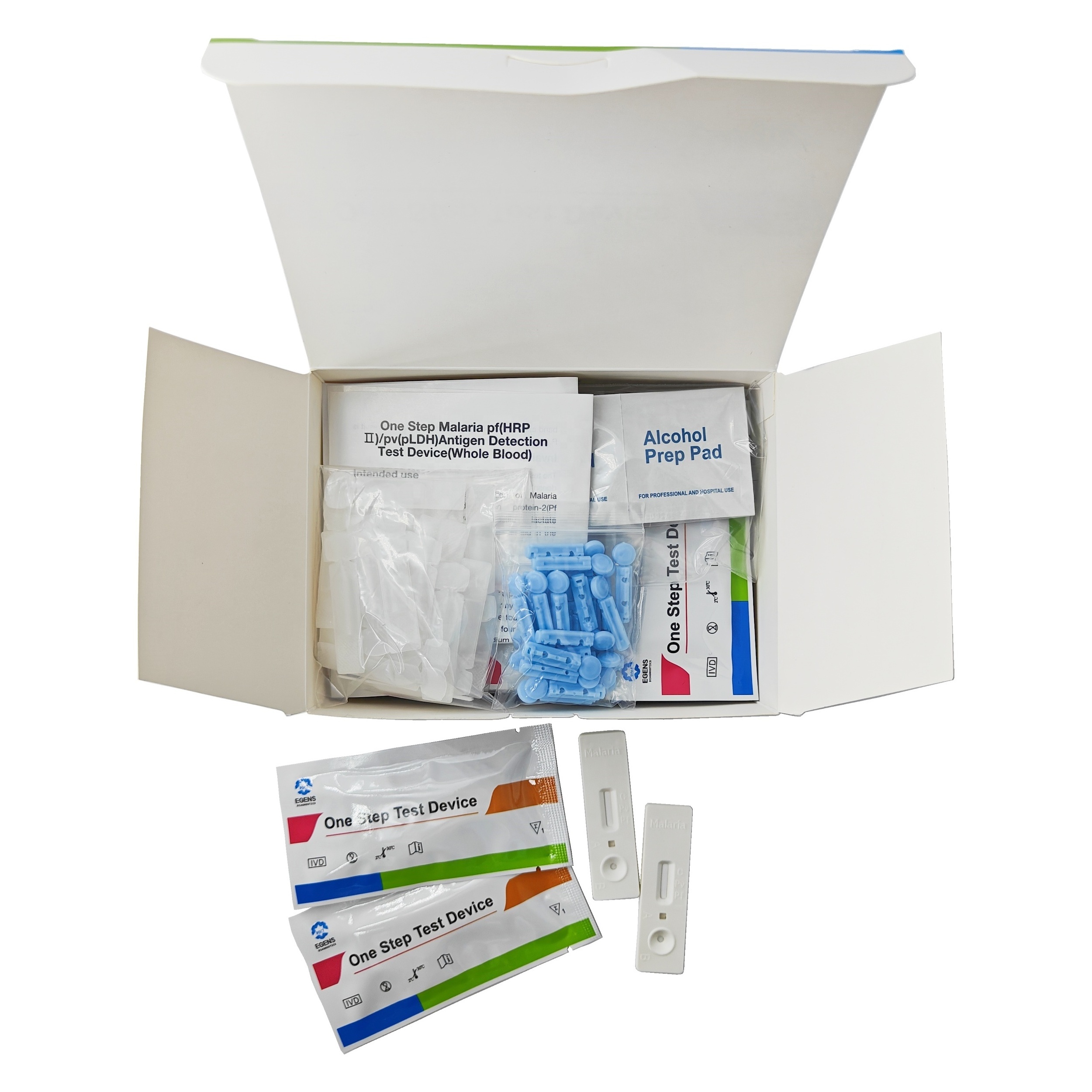
REAGENTS AND MATERIALS PROVIDED
1. Insert Each kit contains 1-50 test devices, each sealed in a foil pouch with three items inside:
a. One cassette device.
b. One pipette dropper.
c. One desiccant.
2. Sample diluent (1-2 bottle, 3 mL)
3. One package insert (instruction for use).
1. Insert Each kit contains 1-50 test devices, each sealed in a foil pouch with three items inside:
a. One cassette device.
b. One pipette dropper.
c. One desiccant.
2. Sample diluent (1-2 bottle, 3 mL)
3. One package insert (instruction for use).
STORAGE AND STABILITY
1. The device can be stored at room temperature (2-30℃6-86℉)。
2. The test device is stable through the expiration date printed on the sealed pouch.
3. The test device must remain in the sealed pouch until use. Do not freeze. Do not uses beyond the expiration date.
1. The device can be stored at room temperature (2-30℃6-86℉)。
2. The test device is stable through the expiration date printed on the sealed pouch.
3. The test device must remain in the sealed pouch until use. Do not freeze. Do not uses beyond the expiration date.
Recommend Products
Hot Searches