- Product Details
- {{item.text}}
Quick Details
-
Brand Name:
-
Pelvifine
-
Model Number:
-
KM530
-
Product name:
-
Pelvic Muscle Trainer
-
Material:
-
medical ABS, silicone, PVC, Stainless steel
-
Function:
-
prevent urinary incontinence; relief pelvic pain
-
Feature:
-
dual channel
-
Certificate:
-
CE/ISO13485
-
Type:
-
Pelvic muscle trainer
-
MOQ:
-
1 pc
-
Packing:
-
1 set/box
-
Keywords:
-
Biofeedback kegel exerciser
-
Service:
-
Online Technology Service
Quick Details
-
Warranty:
-
1 Year
-
After-sale Service:
-
Online technical support
-
Place of Origin:
-
Guangdong, China
-
Brand Name:
-
Pelvifine
-
Model Number:
-
KM530
-
Product name:
-
Pelvic Muscle Trainer
-
Material:
-
medical ABS, silicone, PVC, Stainless steel
-
Function:
-
prevent urinary incontinence; relief pelvic pain
-
Feature:
-
dual channel
-
Certificate:
-
CE/ISO13485
-
Type:
-
Pelvic muscle trainer
-
MOQ:
-
1 pc
-
Packing:
-
1 set/box
-
Keywords:
-
Biofeedback kegel exerciser
-
Service:
-
Online Technology Service
Wholesale price CE certificated biofeedback pelvic floor muscle toner vaginal tightening Kegel muscle exercise stimulator
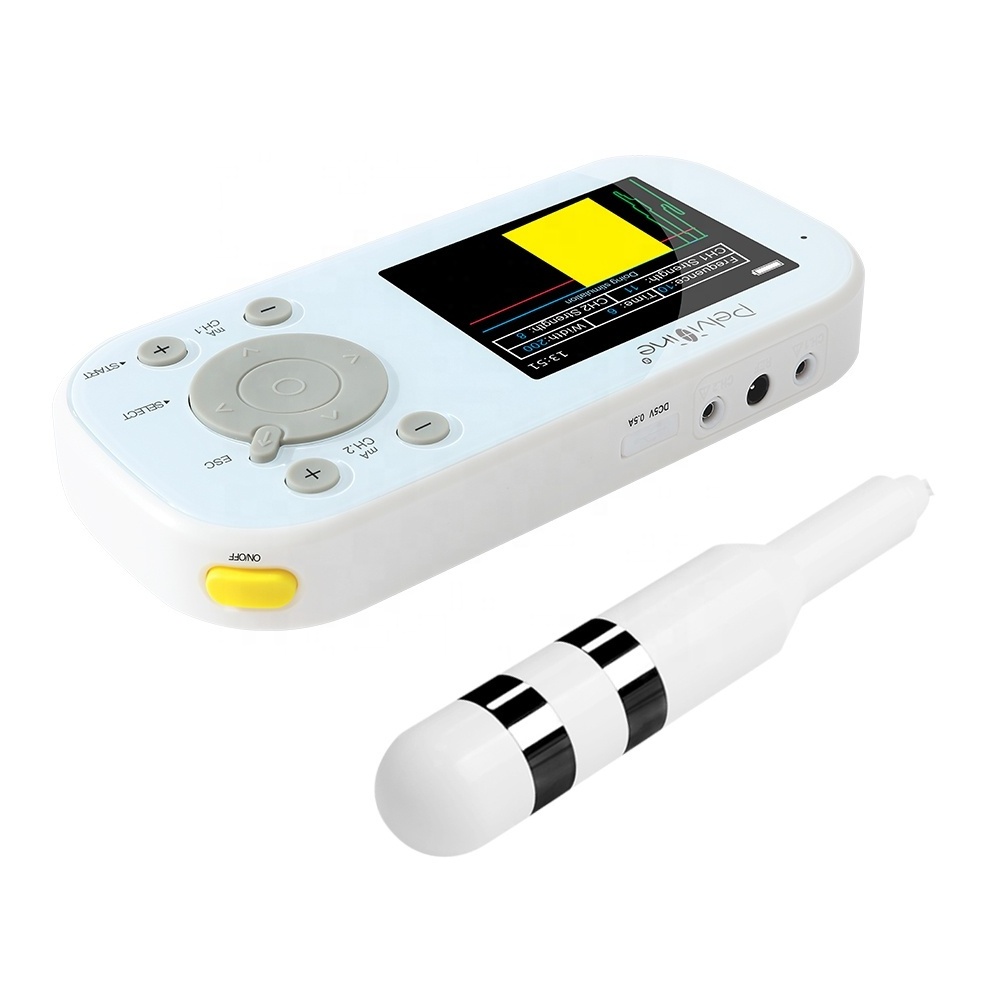
1. Introduction
This Biofeedback device is a new type of biofeedback and neuromuscular electrical stimulation therapy device for patients with muscle dysfunction through the evaluation of myoelectric signal acquisition, multimedia biofeedback training, electromyography triggered electrical stimulation, passive electrical stimulation training and treatment. Perform routine muscle training, combined with individualized electrical stimulation therapy, awaken and activate muscles, accelerate the recovery of muscle tone and elastic, and have a good effect on preventing and treating muscle disorders. Features are as shown below:
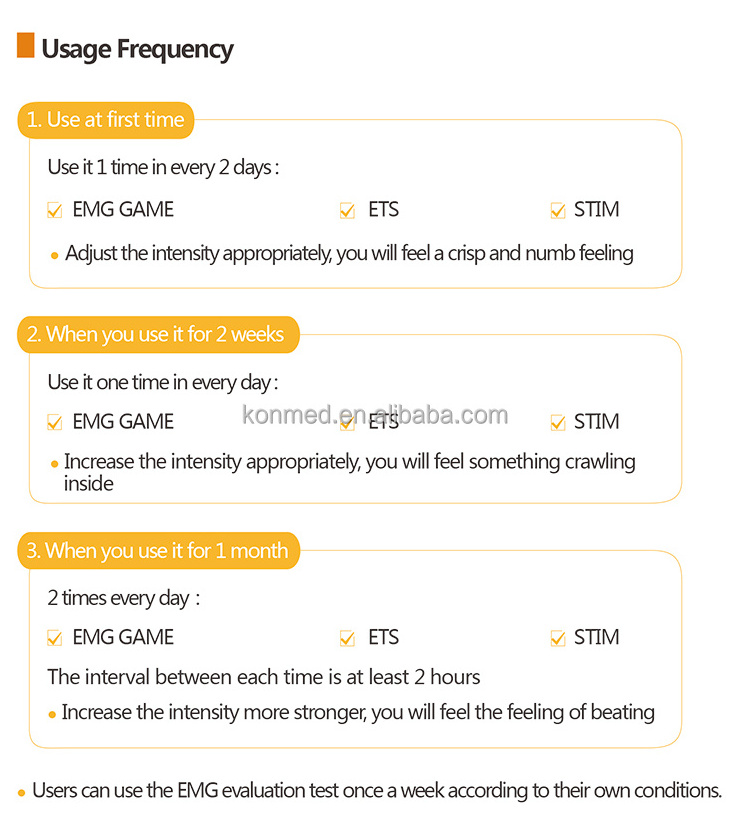
Four operation modes (EMG Test, EMG Game, ETS, and STIM) have been set up to assist patients in exercising.
EMG: Electromyography
ETS: Electromyography triggered stimulation
STIM: Neuromuscular stimulation
Independent dual-channel EMG signals acquisition. EMG data of multiple sites are obtained simultaneously to provide basis for
treatment.
Ergonomic design, effectively prevent the vaginal/anal probe off and rotation, to ensure that treatment effect.
All patient-contacting materials are tested and passed the bio-compatibility according to ISO10993-1 requirements.
This Biofeedback device is a new type of biofeedback and neuromuscular electrical stimulation therapy device for patients with muscle dysfunction through the evaluation of myoelectric signal acquisition, multimedia biofeedback training, electromyography triggered electrical stimulation, passive electrical stimulation training and treatment. Perform routine muscle training, combined with individualized electrical stimulation therapy, awaken and activate muscles, accelerate the recovery of muscle tone and elastic, and have a good effect on preventing and treating muscle disorders. Features are as shown below:
Four operation modes (EMG Test, EMG Game, ETS, and STIM) have been set up to assist patients in exercising.
EMG: Electromyography
ETS: Electromyography triggered stimulation
STIM: Neuromuscular stimulation
Independent dual-channel EMG signals acquisition. EMG data of multiple sites are obtained simultaneously to provide basis for
treatment.
Ergonomic design, effectively prevent the vaginal/anal probe off and rotation, to ensure that treatment effect.
All patient-contacting materials are tested and passed the bio-compatibility according to ISO10993-1 requirements.

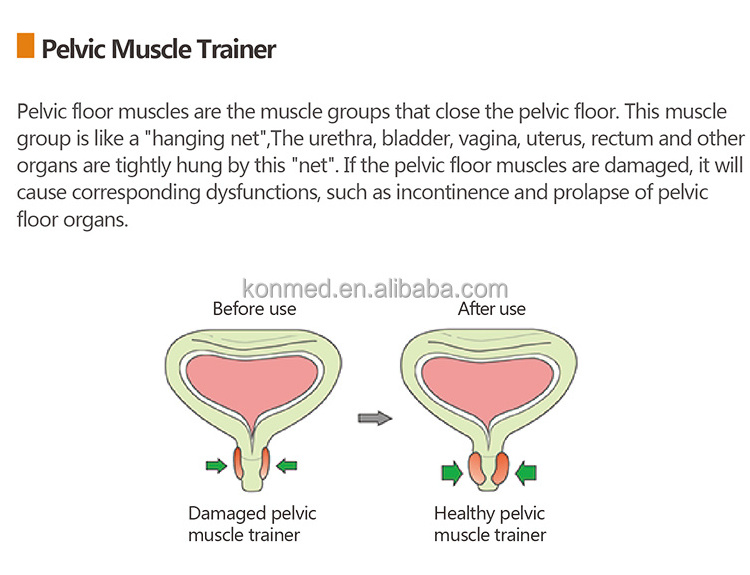
Specification
|
item
|
value
|
|
Place of Origin
|
China
|
|
|
Guangdong
|
|
Brand Name
|
Pelvifine
|
|
Model Number
|
KM530
|
|
Instrument classification
|
Class II
|
|
Warranty
|
1 Year
|
|
After-sale Service
|
Online technical support
|
|
Product name
|
Pelvic Muscle Trainer
|
|
Material
|
medical ABS, silicone, PVC, Stainless steel
|
|
Function
|
prevent urinary incontinence; relief pelvic pain
|
|
Feature
|
dual channel
|
|
Certificate
|
CE/ISO13485
|
|
Type
|
Pelvic muscle trainer
|
|
MOQ
|
1 pc
|
|
Packing
|
1 set/box
|
|
Keywords
|
Biofeedback kegel exerciser
|
|
Service
|
Online Technology Service
|
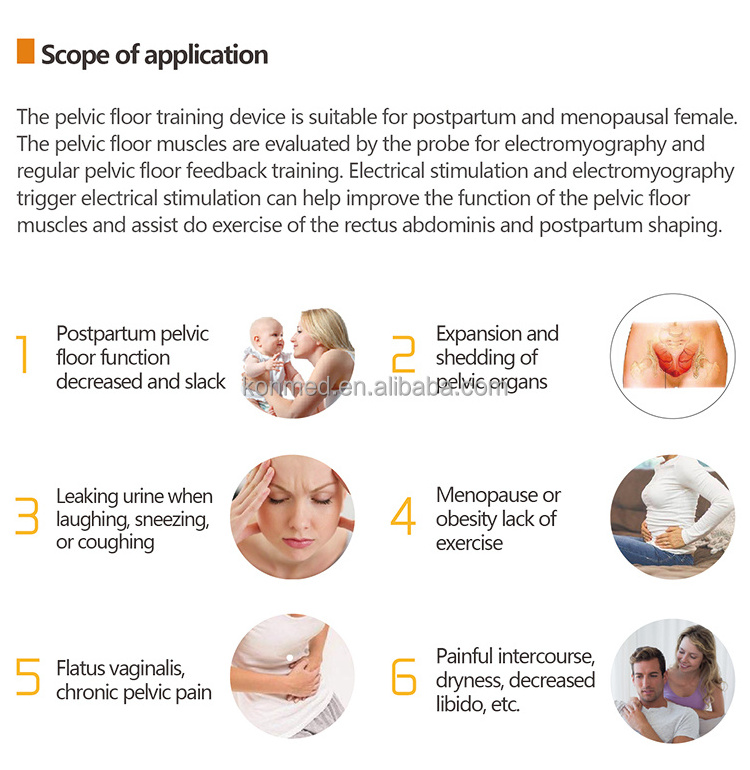
2. Indications for Use
The device is for home use and the intended operator is patient who has muscle dysfunction.
For EMG:
To determine the activation timing of muscles for:
1) Retaining of muscle activation
2) Coordination of muscle activation
An indication of the force produced by muscle for control and maintenance of muscle contractions.
3) Relaxation muscle training
4) Muscle re-education
5) EMG Game for training muscle power, explosive power and speed.
The device is for home use and the intended operator is patient who has muscle dysfunction.
For EMG:
To determine the activation timing of muscles for:
1) Retaining of muscle activation
2) Coordination of muscle activation
An indication of the force produced by muscle for control and maintenance of muscle contractions.
3) Relaxation muscle training
4) Muscle re-education
5) EMG Game for training muscle power, explosive power and speed.
For EMG triggered Stim:
1) Stroke rehab by muscle re-education
2) Relaxation of muscle spasms
3) Prevention or retardation of disuse atrophy
4) Increase local blood circulation
5) Muscle re-education
6) Maintaining or increasing range of motion
1) Stroke rehab by muscle re-education
2) Relaxation of muscle spasms
3) Prevention or retardation of disuse atrophy
4) Increase local blood circulation
5) Muscle re-education
6) Maintaining or increasing range of motion
As nonimplanted electrical continence device:
The device is a non-implanted muscle stimulator designed to provide electrical stimulation and neuromuscular re-education for the purpose of rehabilitation of weak muscles, pelvic floor muscles and restoration of neuromuscular control during the treatment of urinary continence.
The device is a non-implanted muscle stimulator designed to provide electrical stimulation and neuromuscular re-education for the purpose of rehabilitation of weak muscles, pelvic floor muscles and restoration of neuromuscular control during the treatment of urinary continence.
3. Adverse Reactions
These kinds of stimulations have been used for many years to stimulate muscle and nerve fibers to treat a number of muscle and nerve related conditions. Over the last 30 years numerous clinical trials and papers have been written. Patients should stop using the device and should consult with their physicians if they experience adverse reactions from the device.
Package Content
1 Main unit
3 Electrode patches
1 Vaginal probe
1 Anal probe (optional)
2 Electrode wires (white)
1 REF signal wire (black)
1 USB wire
1 User Manual
These kinds of stimulations have been used for many years to stimulate muscle and nerve fibers to treat a number of muscle and nerve related conditions. Over the last 30 years numerous clinical trials and papers have been written. Patients should stop using the device and should consult with their physicians if they experience adverse reactions from the device.
Package Content
1 Main unit
3 Electrode patches
1 Vaginal probe
1 Anal probe (optional)
2 Electrode wires (white)
1 REF signal wire (black)
1 USB wire
1 User Manual
Hot Searches